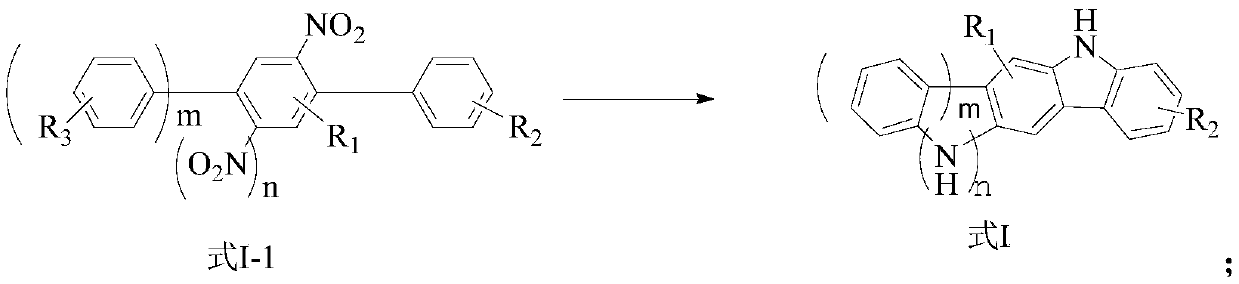
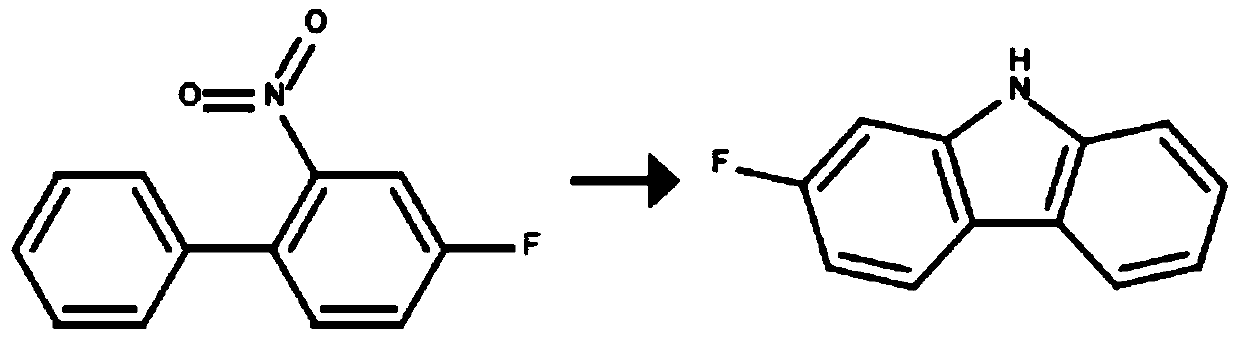
Preparation method of carbazole ring-containing compound
A technology of compounds and carbazole rings, which is applied in the field of preparation of compounds containing carbazole rings, can solve the problems of long reaction time, dangerous solid waste, troublesome disposal, etc., achieve easy post-processing, reduce reaction temperature and reaction time, The effect of reducing dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] In this embodiment, the preparation method of 2-fluorocarbazole is provided, and the reaction formula is as follows:
[0043]
[0044] In a 100ml three-necked flask, add 50ml o-dichlorobenzene, 2.17g (0.01mol) 4-fluoro-2-nitro-1,1'-biphenyl, 2.62g (0.01mol) triphenylphosphine, 1.12g (0.02mol) iron powder was heated to 177°C and refluxed for 20 hours, filtered while it was hot, the mother liquor was concentrated to dryness under reduced pressure, the obtained solid was ground, washed with methanol, and filtered to remove methanol, the obtained solid silica gel column chromatography was separated , sherwood oil: ethyl acetate: dichloromethane=8:1:1 (volume ratio) elution, obtains product 2-fluorocarbazole 1.70 grams, yield 91.9%, the product is carried out with the solariX XR mass spectrometer that BRUKER produces Detect and determine that the product molecular weight is 185.
Embodiment 2
[0046] Compared with Example 1, the only difference is that after adding iron powder, the reaction temperature was kept at 110°C for 20 hours to obtain 1.71 grams of product 2-fluorocarbazole, with a yield of 92.4%. The product was detected by solariX XR produced by BRUKER , determine that the product molecular weight is 185.
Embodiment 3
[0048] Compared with Example 1, the only difference is that the reaction solvent was changed to toluene, and the reaction temperature was kept at 106°C for 10 hours to obtain 1.75 grams of product 2-fluorocarbazole, with a yield of 94.6%, using solariX XR produced by BRUKER The product is detected to determine that the molecular weight of the product is 185.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com