Red solid fluorescent luminescent material and preparation method thereof
A luminescent material, red technology, applied in luminescent materials, chemical instruments and methods, organic chemistry, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
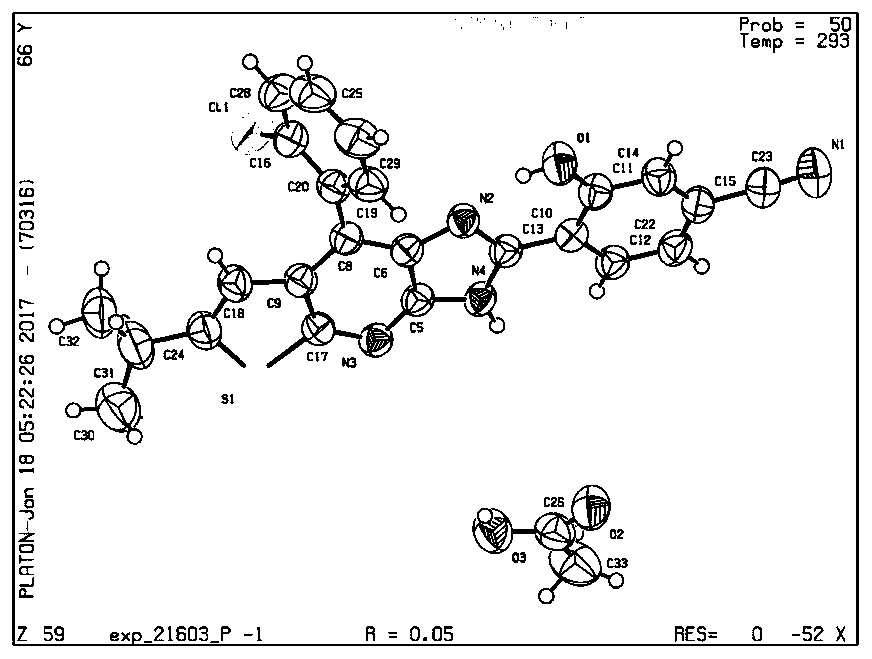
[0024] Embodiment 1: the synthesis of compound CA1
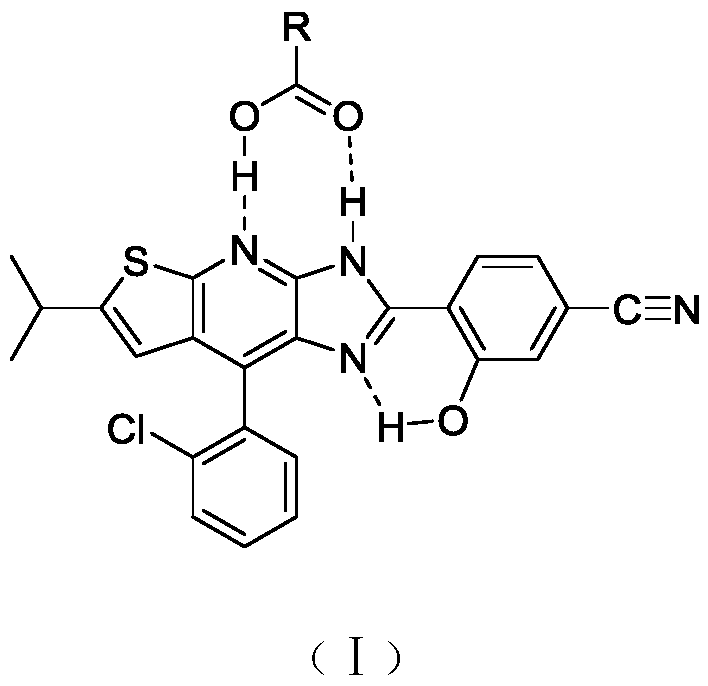
[0025] 4-(8-(2-chlorophenyl)-6-isopropyl-3H-imidazol[4,5-b]thiophene[3,2-e]pyridine)-3- Hydroxybenzonitrile (22mg, 0.05mmol) was dissolved in dichloromethane (5.0mL), and acetic acid (300mg, 5mmol) was added to the resulting solution at room temperature, and slowly volatilized at room temperature, filtered by suction to obtain the target carboxylic acid Salt.
Embodiment 2
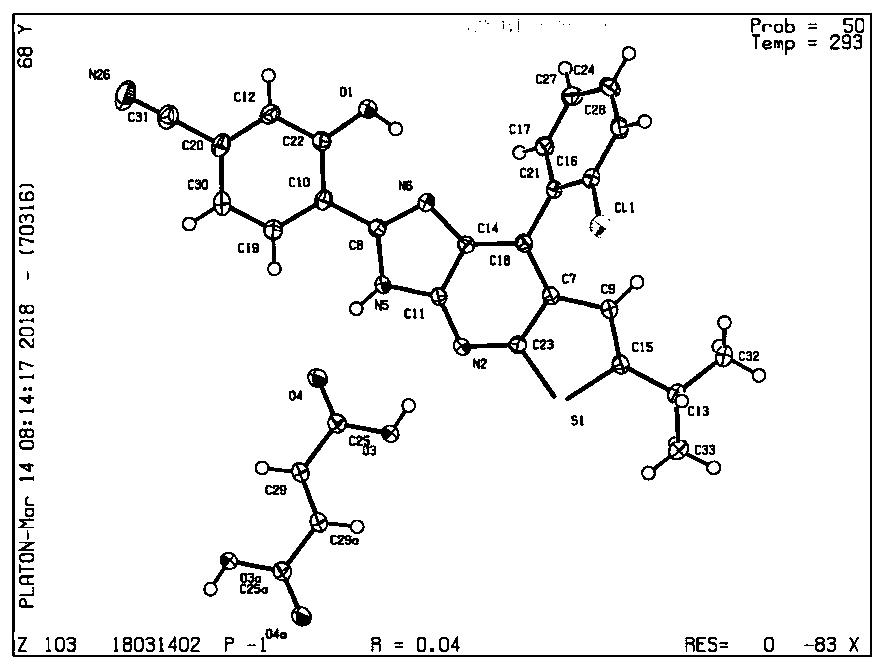
[0026] Embodiment 2: the synthesis of compound CA2
[0027] Synthetic method reference example 1, 4-(8-(2-chlorophenyl)-6-isopropyl-3H-imidazol[4,5-b]thiophene[3,2-e]pyridine)-3-hydroxyl Benzonitrile (22 mg, 0.05 mmol), tetrahydrofuran (0.5 mL), butyric acid (88 mg, 1 mmol).
Embodiment 3
[0028] Embodiment 3: the synthesis of compound CA3
[0029] Synthetic method reference example 1, 4-(8-(2-chlorophenyl)-6-isopropyl-3H-imidazol[4,5-b]thiophene[3,2-e]pyridine)-3-hydroxyl Benzonitrile (22 mg, 0.05 mmol), chloroform (1.0 mL), chloroacetic acid (283 mg, 3 mmol).
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap