Febrile convulsion resisting benzodiazepine pharmaceutical composition and intelligent transdermal delivery system thereof
A technology for transdermal delivery of benzodiazepines, applied in the field of biomedicine, can solve the problems of inappropriate iontophoresis transdermal drug delivery, poor water solubility, etc., and achieve the effect of facilitating remote control of drug delivery
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
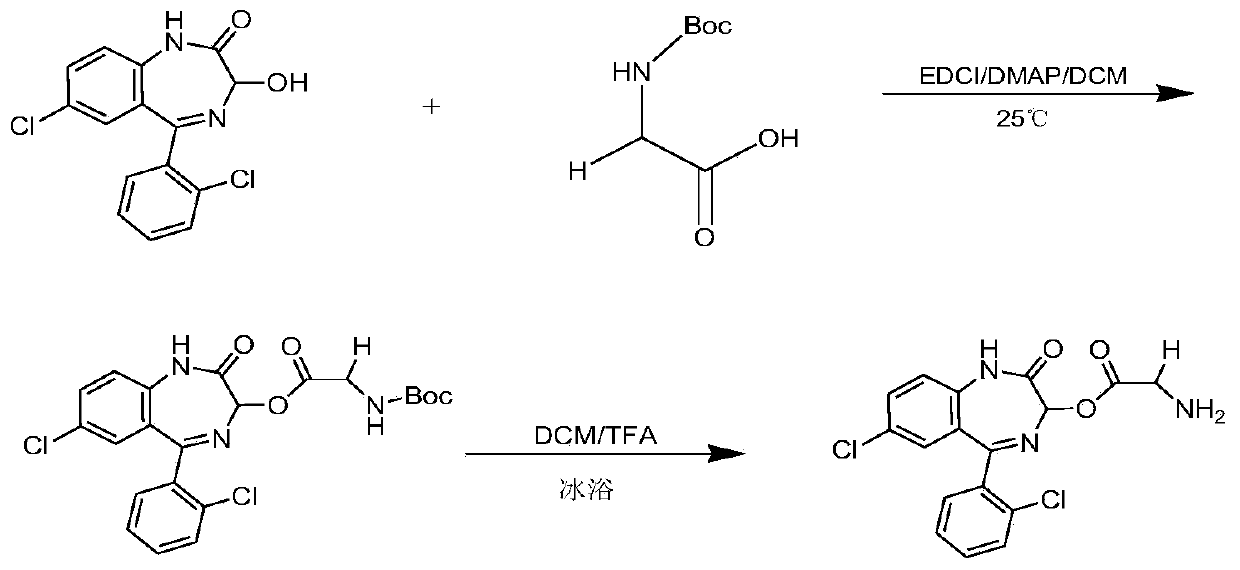
[0047] Synthesis of LZP-Gly: Weigh lorazepam (LZP, 0.50g, 1.56mmol), 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDCI, 0.48g, 3.10mmol), 4-dimethylaminopyridine (DMAP, 0.10g, 0.78mmol), Boc-glycine (Boc-Gly, 0.33g, 1.89mmol), dissolved in 30ml of dichloromethane (DCM), magnetically stirred at room temperature Stir overnight. The reaction was monitored by thin layer chromatography. After the reaction was completed, the precipitate was removed by filtration, and the solution was spin-dried. The residue was redissolved with ethyl acetate and washed with water (2×30ml), saturated NaHCO 3 The solution (2 x 30ml) was washed successively with saturated NaCl solution (1 x 30ml). Anhydrous MgSO for organic layer 4 Dry, filter, and spin dry the solvent. The product was purified by silica gel column chromatography (petroleum ether: ethyl acetate = 4:1). Collect the eluate containing the product and spin dry. Take the purified substance in a flask, add 5ml DCM and 5...
Embodiment 2
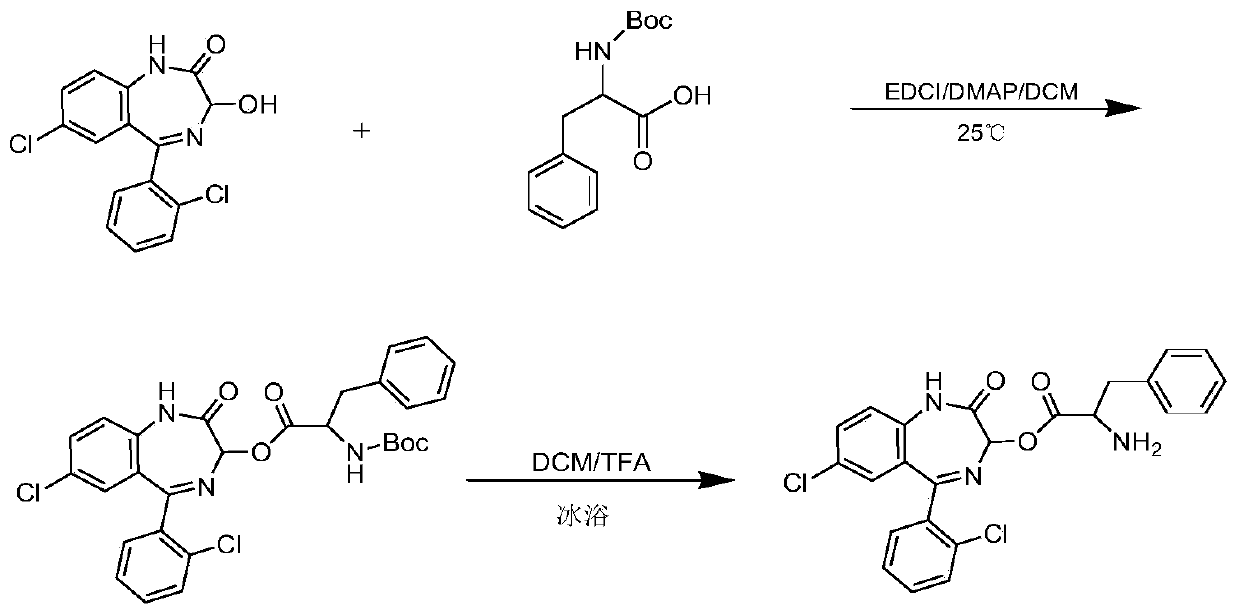
[0050] Synthesis of LZP-Phe: Weigh LZP (0.77g, 2.40mmol), EDCI (0.75g, 4.84mmol), DMAP (0.15g, 1.23mmol) and Boc-phenylalanine (Boc-Phe, 0.77g, 2.91mmol ), dissolved in 30ml of DCM, and stirred overnight with magnetic stirring at room temperature. The reaction was monitored by thin layer chromatography. After the reaction was completed, the precipitate was removed by filtration, and the solution was spin-dried. The residue was redissolved with ethyl acetate and washed with water (2×30ml), saturated NaHCO 3 The solution (2 x 30ml) was washed successively with saturated NaCl solution (1 x 30ml). Anhydrous MgSO for organic layer 4 Dry, filter, and spin dry the solvent. The product was purified by silica gel column chromatography (petroleum ether: ethyl acetate = 3:1). Collect the eluate containing the product and spin dry. Take the purified substance in a flask, add 5ml DCM and 5ml TFA in sequence, and stir magnetically for 2h under ice bath conditions. After the solvent w...
Embodiment 3
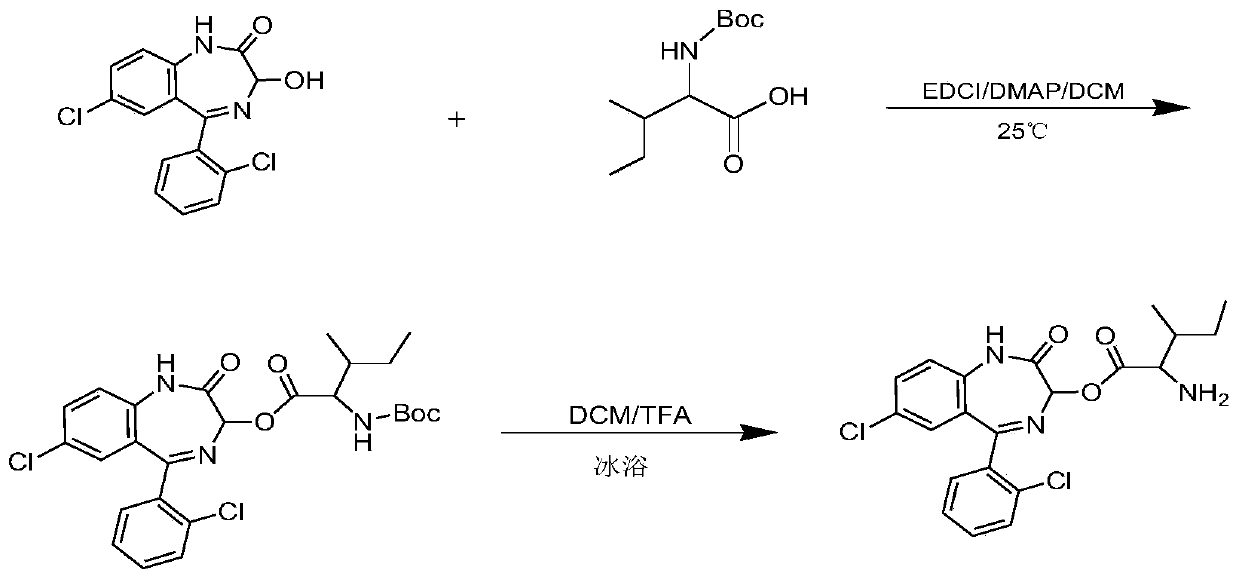
[0053] Synthesis of LZP-Ile: Weigh LZP (0.90g, 2.80mmol), EDCI (0.90g, 5.61mmol), DMAP (0.17g, 1.39mmol) and Boc-isoleucine (Boc-Ile, 0.79g, 3.40mmol ), dissolved in 30ml of dichloromethane (DCM), and stirred overnight with magnetic stirring at room temperature. The reaction was monitored by thin layer chromatography. After the reaction was completed, the precipitate was removed by filtration, and the solution was spin-dried. The residue was redissolved with ethyl acetate and washed with water (2×30ml), saturated NaHCO 3The solution (2 x 30ml) was washed successively with saturated NaCl solution (1 x 30ml). Anhydrous MgSO for organic layer 4 Dry, filter, and spin dry the solvent. The product was purified by silica gel column chromatography (petroleum ether: ethyl acetate = 3:1). Collect the eluate containing the product and spin dry. Take the purified substance in a flask, add 5ml DCM and 5ml TFA in sequence, and stir magnetically for 2h under ice bath conditions. After...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com