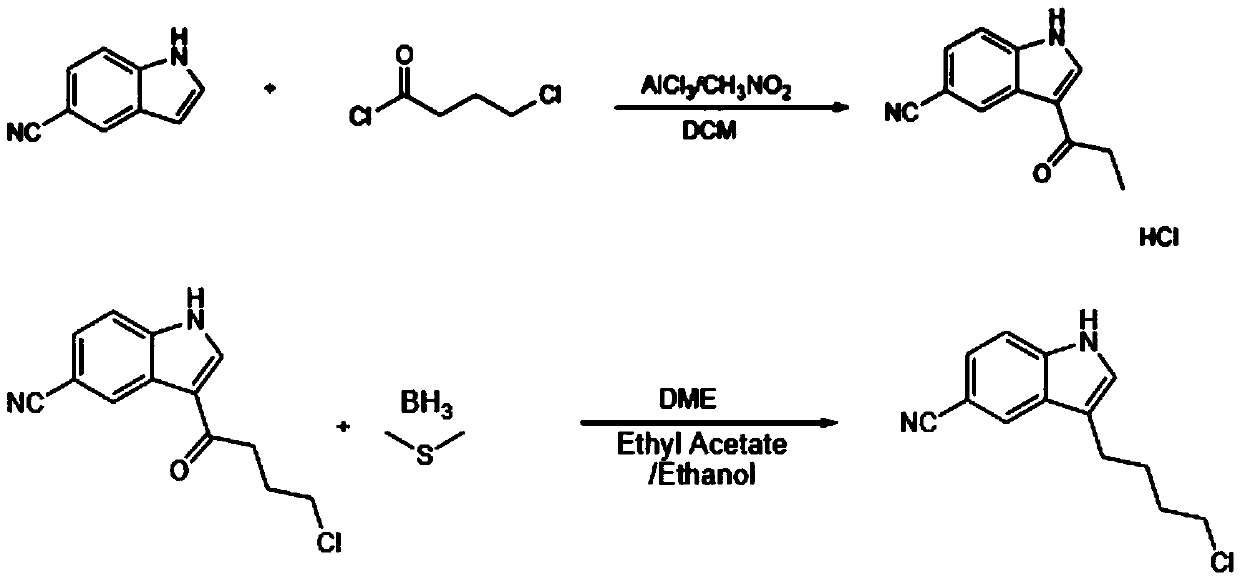
Production process of 3-(4-chlorobutyl)indole-5-carbonitrile
A production process, chlorobutyl technology, applied in the field of preparation of pharmaceutical intermediates, can solve the problems of no implementation value and social and economic benefits, increased reaction time, complex synthesis process conditions, etc., to achieve maximum implementation value and social and economic benefits, The effect of improving the yield and product purity and simplifying the synthesis process conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] A kind of production technology of 3-(4-chlorobutyl) indole-5-carbonitrile comprises the steps: Step 1: 450ml of dichloromethane is charged into a clean reactor, and nitromethane is charged into the same reaction Replace the reactor with nitrogen once, protect the reactor with nitrogen, stir and cool it, add 40g of aluminum chloride to the same reactor, keep the temperature at 5°C, drop it gently, put 45g of 4-chlorobutyl chloride into the same reaction In the container, keep the temperature at 5°C, stir evenly, and cool down;
[0024] Step 2: Put 5-cyanindole in another clean reactor, stir until it is completely dissolved in the solvent, transfer the solution to a clean high-level tank, and gently fill the solution into a sheathed water-permeable In the reactor, store at 5°C, stir for 2 hours, heat to 20°C, let stand for 1 hour, cool down, transfer the reactant to another reactor, heat to 20°C, peel for 1 hour, stand for freezing, Centrifuge, wash the wet filter cake ...
Embodiment 2
[0037] A kind of production technology of 3-(4-chlorobutyl) indole-5-carbonitrile comprises the steps: Step 1: 450ml of dichloromethane is charged into a clean reactor, and nitromethane is charged into the same reaction Replace the reactor with nitrogen once, protect the reactor with nitrogen, stir and cool it, add 40g of aluminum chloride to the same reactor, keep the temperature at 5°C, drop it gently, put 45g of 4-chlorobutyl chloride into the same reaction In the container, keep the temperature at 10°C, stir evenly, and cool down;
[0038] Step 2: Put 5-cyanindole in another clean reactor, stir until it is completely dissolved in the solvent, transfer the solution to a clean high-level tank, and gently fill the solution into a sheathed water-permeable In the reactor, store at 8°C, stir for 2 hours, heat to 25°C, stand for 1 hour, cool, transfer the reactant to another reactor, heat to 25°C, peel for 1 hour, stand for freezing, Centrifuge, wash the wet filter cake with wat...
Embodiment 3
[0051] A kind of production technology of 3-(4-chlorobutyl) indole-5-carbonitrile comprises the steps: Step 1: 450ml of dichloromethane is charged into a clean reactor, and nitromethane is charged into the same reaction Replace the reactor with nitrogen once, protect the reactor with nitrogen, stir and cool it, add 40g of aluminum chloride to the same reactor, keep the temperature at 5°C, drop it gently, put 45g of 4-chlorobutyl chloride into the same reaction In the container, keep the temperature at 8°C, stir evenly, and cool down;
[0052] Step 2: Put 5-cyanindole in another clean reactor, stir until it is completely dissolved in the solvent, transfer the solution to a clean high-level tank, and gently fill the solution into a sheathed water-permeable In the reactor, store at 6°C, stir for 2 hours, heat to 22°C, let stand for 1 hour, cool down, transfer the reactant to another reactor, heat to 22°C, peel for 1 hour, stand for freezing, Centrifuge, wash the wet filter cake ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com