Skin tissue immune cell in-situ detection kit for lupus erythematosus typing and application thereof
A technology for lupus erythematosus and immune cells, which is applied in the field of immunoassays, and can solve the problems of difficulty in obtaining, easy to pollute and degrade, and small volume of samples
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] Example 1: Preparation method of skin tissue in situ multi-label immunostaining kit
[0084] The results of multi-label immunostaining are captured through the processing of filters of four color channels (DAPI, FITC, Cy3, Cy5), and there will be some overlap between the emission spectra of different filters. The multi-spectral analysis software is based on the peak of the spectrum when identifying and splitting the spectrum. When multiple monoclonal antibodies are co-stained on a tissue section, the spectra corresponding to multiple antibodies will overlap to varying degrees. When an antibody develops too much color, its chromogenic spectrum may cover the spectrum of adjacent antibodies, resulting in cross-coloring between two or more antibodies. In order to avoid this situation, in the preparation of the skin tissue in situ multi-label immunostaining kit, the condition optimization of each key step is very important.
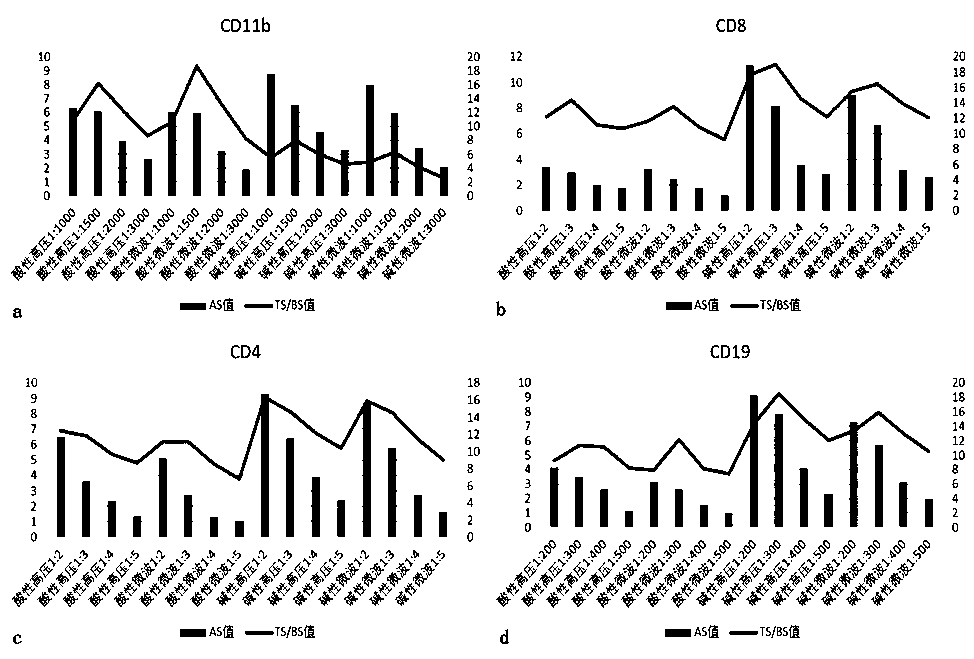
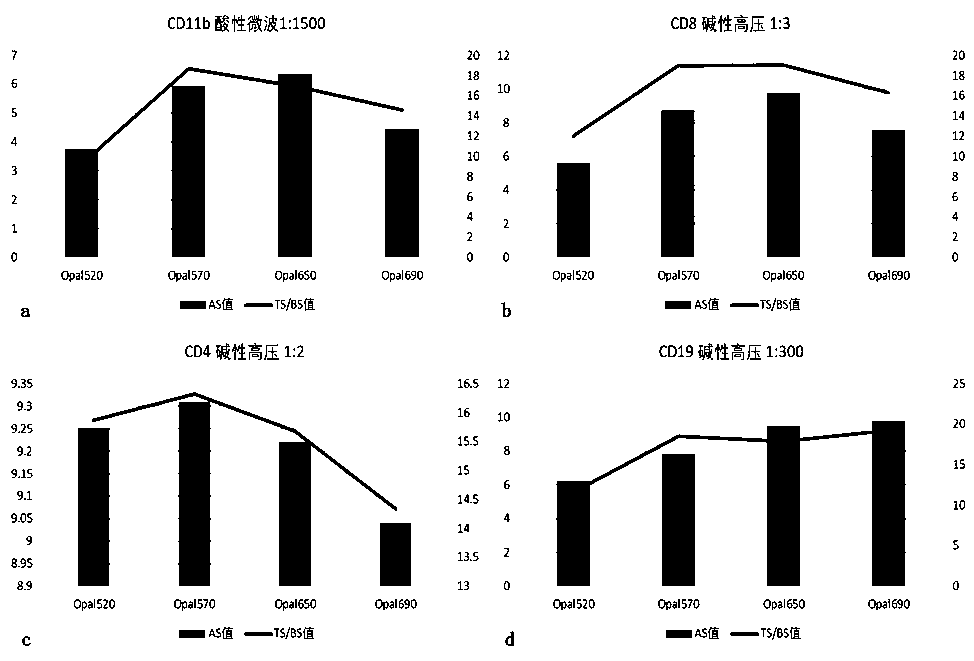
[0085] 1.1 Determine the optimal antigen retriev...
Embodiment 2
[0137] Example 2: The method of using the skin tissue in situ multi-marker immunostaining kit.
[0138] 2.1 Pretreatment of paraffin sections
[0139] Select paraffin sections of skin tissue and bake them at 60°C for 2 hours; take them out after 2 hours, soak them in preheated turpentine oil for 15 minutes, then soak them in 100% ethanol, 95% ethanol, 70% ethanol, and sterilized water for 10 minutes. minute.
[0140] 2.2 The first round of staining: CD11b-Opal570
[0141] 1) Take 200ml of acidic restoration solution (pH6.0), put it into the antigen restoration box containing slices, put the antigen restoration box into the microwave oven, adjust to high heat, and heat for 2-3min (depending on the operating environment and usage The electric appliance brand is different, and the determination of the time depends on the observation of the boiling of the liquid surface in the repair box), when the repair liquid in the repair box starts to boil, quickly adjust to the low fire ge...
Embodiment 3
[0214] Example 3: Determination of detection indicators of skin tissue in situ multi-label immunostaining kit that can assist in the diagnosis and typing of lupus erythematosus
[0215] 3.1 Distinguish between normal people and lupus
[0216] 1) Select 18 cases of DLE, SLE, and SCLE skin lesions from different patients, and select 45 cases of normal human skin as a control, and use the skin tissue in situ multi-label immunostaining kit for sample detection to obtain corresponding data;
[0217] 2) Use IBM SPSS Statistics 23 to build a ROC analysis model, set the data of 54 lupus patients as the case group LE, and the data of 45 normal subjects as the normal control NC;
[0218] 3) CD4 single positive test results (CD4 / DAPI), CD8 single positive test results (CD8 / DAPI), CD19 single positive test results (CD19 / DAPI), CD11b single positive test results (CD11b / DAPI) , CD4-CD8 dual detection results ((CD4+CD8) / DAPI), CD4-CD19 dual detection results ((CD4+CD19) / DAPI), CD4-CD11b dua...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com