Pneumococcal polysaccharides and their use in immunogenic polysaccharide-carrier protein conjugates
A protein conjugate, immunogenic technology, which can be used in multivalent vaccines, active ingredients of heterocyclic compounds, and medical preparations of non-active ingredients, etc., and can solve problems such as the increase in the prevalence of pneumococcus
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
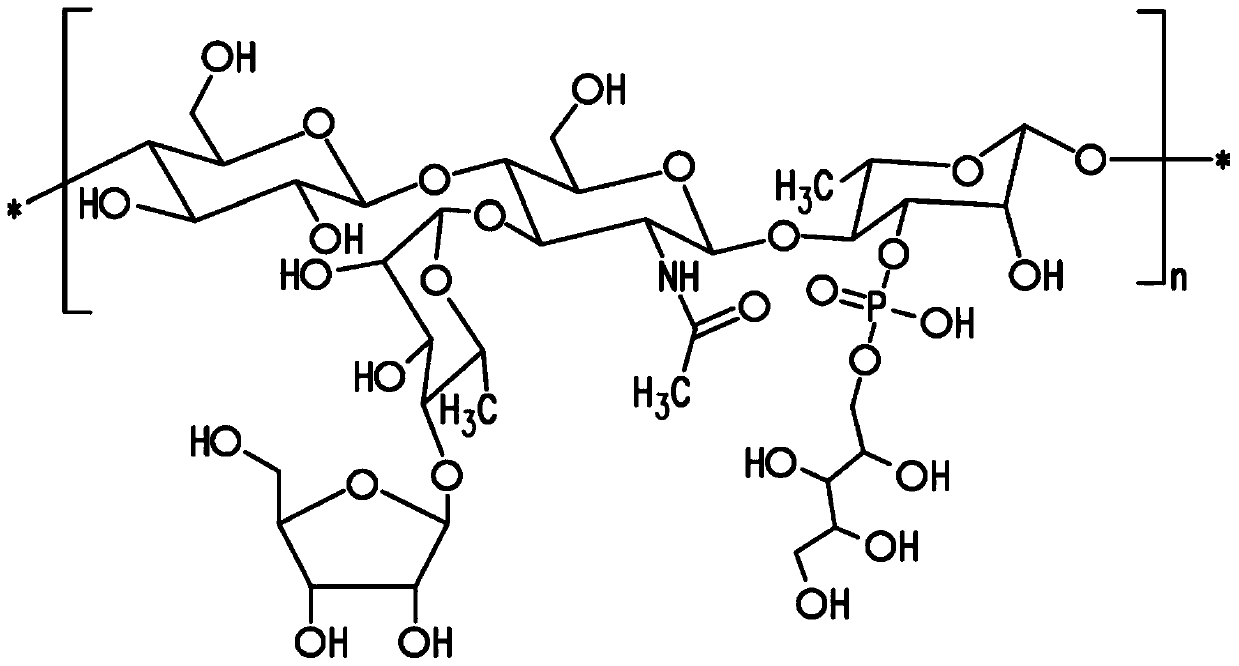
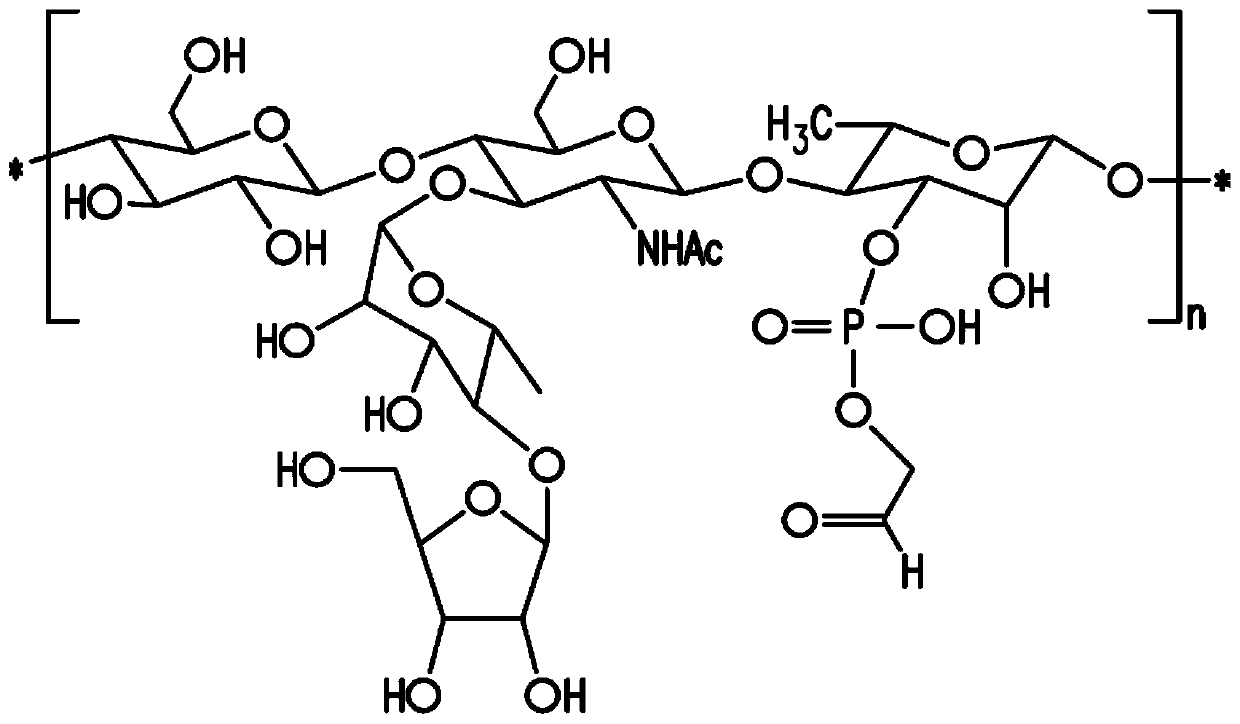
[0158] Embodiment 1: Preparation of Streptococcus pneumoniae capsular polysaccharide
[0159] Methods of culturing pneumococci are well known in the art. See, eg, Chase, 1967, Methods of Immunology and Immunochemistry 1:52. Methods of preparing pneumococcal capsular polysaccharides are also well known in the art. See eg European Patent No. EP 0 497 524 B1. The method described below generally follows that described in European Patent No. EP 0497 524 B1 and is generally applicable to all pneumococcal serotypes unless specifically modified.
[0160] Isolates of pneumococcal subtype 24F were obtained from the Merck Culture Collection. Subtypes can be differentiated based on the Quelling reaction using specific antisera if desired. See, eg, US Patent No. 5,847,112. The isolates obtained from the clonal isolation were further clonally isolated by sequential plating in two stages on agar plates consisting of an animal component-free medium containing soy peptone, yeast extract ...
Embodiment 2
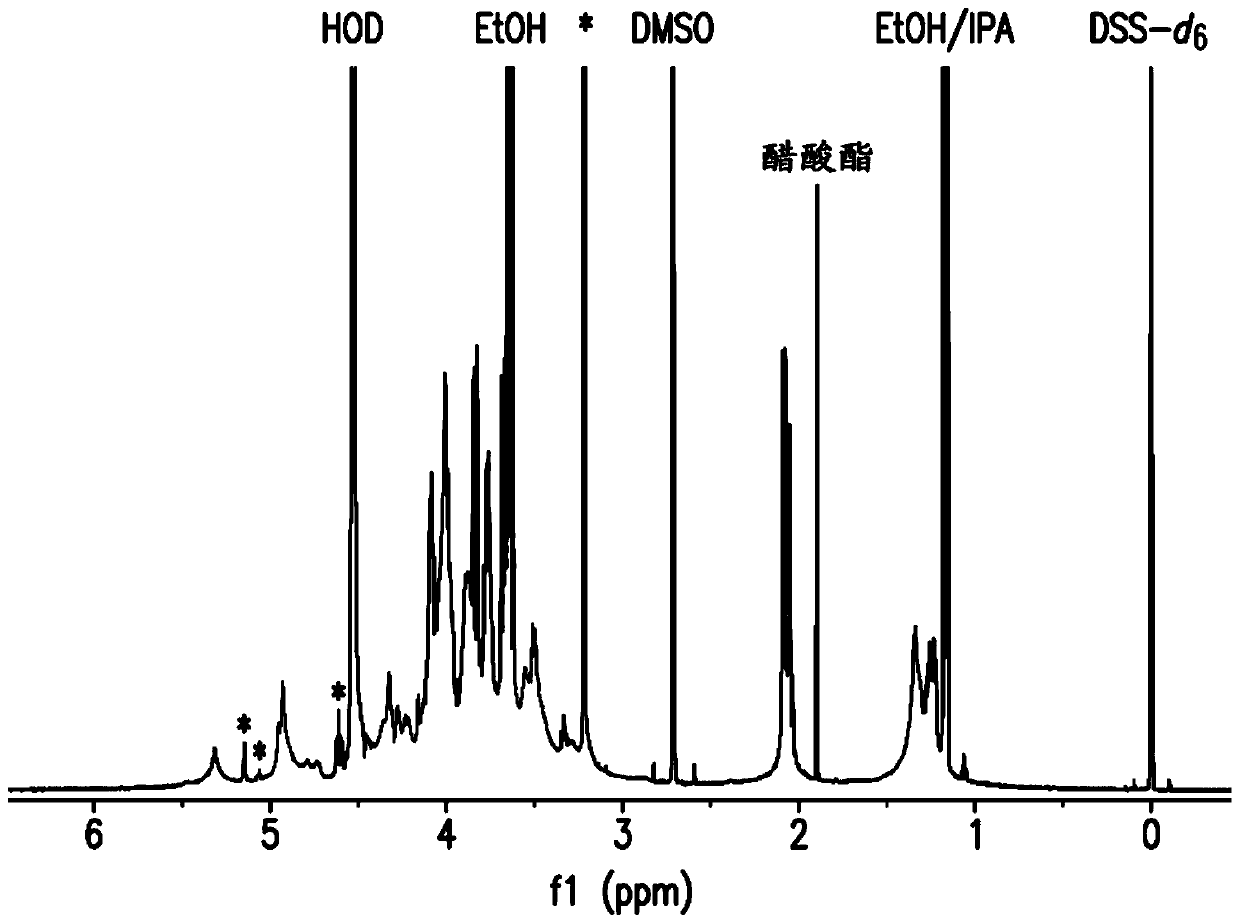
[0167] Embodiment 2: NMR structure analysis of polysaccharide
[0168] The strategy for determining the structure of polysaccharides involves a multi-step process performed essentially as described by Abeygunawardana et al., Determination of the Chemical Structure of Complex Polysaccharides by Heteronuclear NMR Spectroscopy in Advances in Biophysical Chemistry 1993, Vol 3, pages 199-249, JAI Press Inc. The purified polysaccharides were examined using standard 1D and 2D NMR techniques. Finally, use 31 P NMR detects the presence of phosphate esters in polysaccharides.
[0169] Monosaccharide residue assignment (assignment) by 1 H- 1 H COZY, double quantum filtering homonuclear COZY and total correlation spectroscopy (TOCSY) were performed. Determined by the combination of heteronuclear single quantum coherence spectroscopy (HSQC) and HSQC-TOCSY 13 C chemical shift. Multiple edited HSQC was used to distinguish methylene from methine. Inter-residue linkage was determined by...
Embodiment 3
[0183] Example 3: Conjugation of S. pneumoniae serotype 24F polysaccharide to CRM197 using reductive amination in dimethylsulfoxide
[0184] Polysaccharides are dissolved, sized to target molecular weight, chemically activated and buffer exchanged by ultrafiltration. The activated polysaccharide and purified CRM197 were separately lyophilized and then redissolved in dimethyl sulfoxide (DMSO). The redissolved polysaccharide and CRM197 solutions were then combined and conjugated as described below. The resulting conjugate was purified by ultrafiltration before a final 0.2 micron filtration. Multiple process parameters such as pH, temperature, concentration and time are controlled in each step to produce conjugates with desired properties.
[0185] Polysaccharide size reduction and oxidation
[0186] The purified pneumococcal capsular Ps powder was dissolved in water and subjected to 0.45 micron filtration. The size of the dissolved polysaccharides was reduced by adding aceti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com