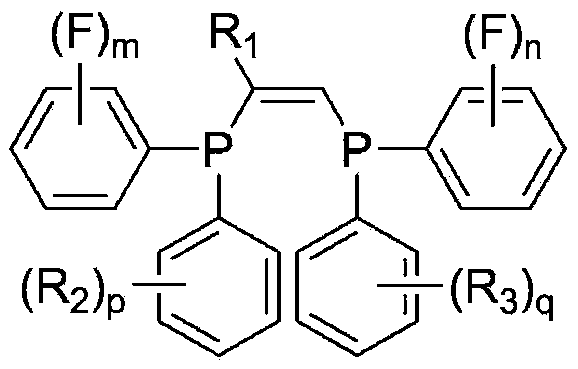
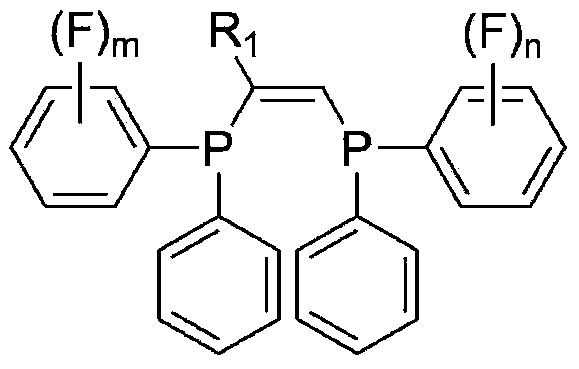
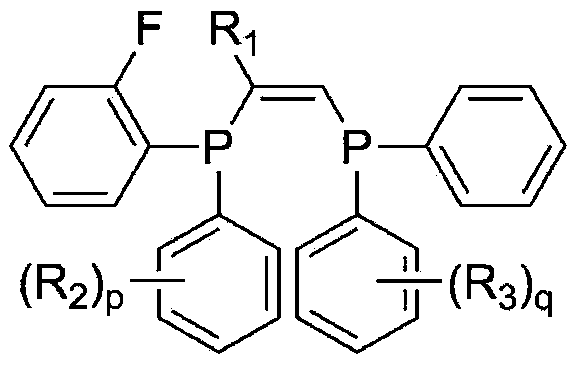
Ligand, oligomerization catalyst comprising same, and method for producing ethylene oligomer by using oligomerization catalyst
一种乙烯低聚物、乙烯低聚的技术,应用在催化剂、有机化合物/氢化物/配位配合物催化剂、碳化合物催化剂等方向,能够解决产量和选择性降低、堵塞和结垢、催化活性降低等问题,达到催化活性和选择性优异、节省成本、催化活性优异的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0114] According to an exemplary embodiment of the present invention, organoaluminoxanes suitable for use as activators in ethylene oligomerization catalyst compositions are oligomeric aluminoxanes which can be prepared by adding water to an aluminum alkyl compound such as trimethylaluminum. compound. The resulting aluminoxane oligomeric compounds may be linear, cyclic or cage compounds, or mixtures thereof.
[0115] The organoaluminoxane may be selected from alkylaluminoxanes such as methylaluminoxane (MAO), ethylaluminoxane (EAO), tetraisobutylaluminoxane (TIBAO) and isobutylaluminoxane ( IBAO), may also be selected from modified alkylaluminoxanes, such as modified methylaluminoxane (MMAO). Modified methylalumoxanes (manufactured by Akzo Nobel N.V.) contain mixed alkyl groups such as isobutyl or n-octyl groups in addition to methyl groups. As a specific example, the organoaluminoxane may be selected from methylalumoxane (MAO), modified methylalumoxane (MMAO), ethylalumoxan...
preparation example A
[0133] [Preparation Example A] Preparation of chloro(2-fluorophenyl)phenylphosphine
[0134]
[0135] Preparation of N,N-Diethylaminochloro(phenyl)phosphine
[0136] N,N-Diethylaminochloro(phenyl)phosphine was prepared by referring to known literature (M.Oliana et.al., J.Org.Chem., 2006, p.2472-2479).
[0137] In a dry flask under nitrogen atmosphere, dichloro(phenyl)phosphine (8.949 g, 50 mmol) was dissolved in n-hexane (400 mL), then diethylamine (7.314 g, 100 mmol) was added slowly at room temperature. The mixture was reacted for more than 1 hour, and then filtered through celite under reduced pressure to remove volatile materials, thereby obtaining the title compound (10.2 g, 94.6%) as a clear colorless liquid.
[0138] 1 H NMR (500MHz, C 6 D. 6 )δ7.74 (m, 2H, aromatic compounds), 7.10 (m, 2H, aromatic compounds), 7.03 (m, 1H, aromatic compounds), 2.85 (m, 4H, -CH 2 -),0.79(m,6H,-CH 3 ).
[0139] Preparation of N,N-diethyl-1-(2-fluorophenyl)-1-phenylphosphinea...
preparation example B
[0145] [Preparation Example B] Preparation of (2-fluorophenyl) (phenyl) phosphine
[0146]
[0147] In a dry flask under a nitrogen atmosphere, the chloro(2-fluorophenyl)phenylphosphine (2.3863 g, 10 mmol) obtained in Preparation A was dissolved in n-hexane (20 mL), and then trimethyltin hydride was slowly added (4.0163 g, 11 mmol). The mixture was reacted for 30 minutes, and then filtered through celite under reduced pressure to remove volatile materials to obtain the title compound (2.0418 g, 100%) as a liquid.
[0148] 1 H NMR (500MHz, C 6 D. 6 )δ7.37 (m, 2H, aromatic compounds), 7.11 (m, 1H, aromatic compounds), 6.97 (m, 3H, aromatic compounds), 6.80 (m, 1H, aromatic compounds), 6.68 (m , 1H, aromatic compounds), 5.51-5.07 (d, 1H, -P).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com