Simple method for preparing ellipticine or substituted ellipticine
A technology for ellipticine and a preparation process, which is applied in the production of bulk chemicals, organic chemistry, etc., can solve the problems of low total yield, harsh reaction conditions, and difficulty in obtaining raw materials, and achieves simple and convenient process operation and high total yield. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] The preparation of embodiment 1 ellipticine
[0040] Preparation of Intermediate 2
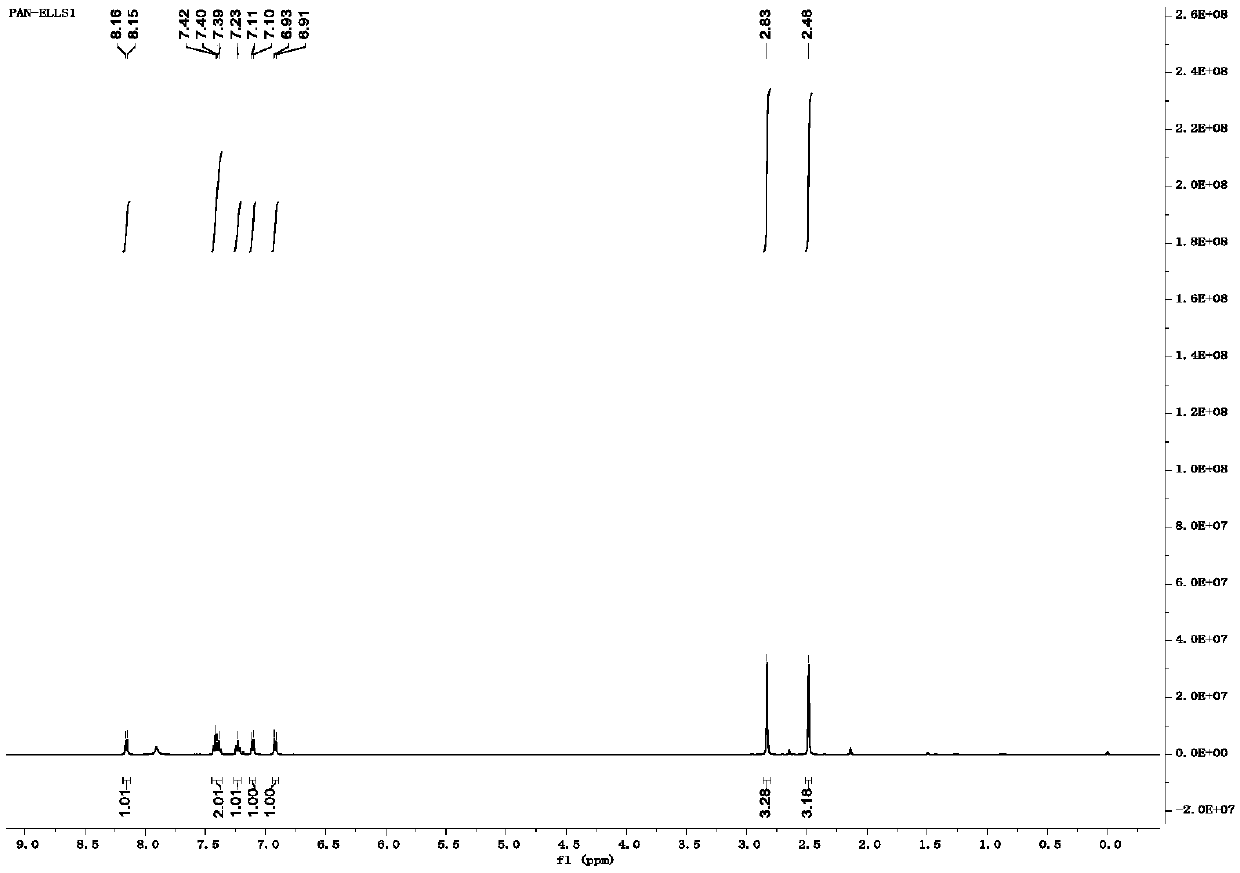
[0041] Add 11.7g (0.1mol) indole and 11.4g (0.1mol) 2,5-hexanedione in a round-bottomed flask, mix and stir until dissolved, add 0.03 equivalents of montmorillonite powder loaded with p-toluenesulfonic acid in advance, in React in a microwave reactor for 10 min. The reaction solution was cooled to room temperature, diluted with ethyl acetate, and the montmorillonite catalyst was filtered off. The solid was washed with ethyl acetate, and the organic phase was washed with saturated sodium carbonate solution, purified water, and saturated sodium chloride solution, and dried over anhydrous sodium sulfate. overnight. Concentrate to obtain 17.5 g of white solid, yield 90.0%. m.p.92-93℃. 1 H NMR (500MHz, CDCl 3 )δ8.16(d, J=7.8Hz, 1H), 7.45-7.36(m, 2H), 7.23(s, 1H), 7.11(d, J=7.2Hz, 1H), 6.92(d, J=7.2 Hz,1H),2.83(s,3H),2.48(s,3H)( figure 1 ).
[0042] Preparation of Intermediate 3
[0...
Embodiment 2
[0050] The preparation of embodiment 2 9-methoxy ellipticine
[0051]
[0052] Preparation of Intermediate 8
[0053] Add 14.7g (0.1mol) of 5-methoxyindole and 13.5g (0.1mol) of 2,5-hexanedione into a round-bottomed flask, mix and stir until dissolved, add 0.03 equivalents of Mongolian pre-loaded with p-toluenesulfonic acid Destoned powder, react in microwave reactor for 10min. The reaction solution was cooled to room temperature, diluted with ethyl acetate, and the montmorillonite catalyst was filtered off. The solid was washed with ethyl acetate, and the organic phase was washed with saturated sodium carbonate solution, purified water, and saturated sodium chloride solution, and dried over anhydrous sodium sulfate. overnight. Concentration gave 8 as a white solid (19.7 g, 87.6% yield, melting point 135-137°C).
[0054] Preparation of Intermediate 9
[0055] Add 3.85g (28.5mmol) of N-methyl-N-phenylformamide into the three-necked flask, slowly add 4.62g (29.5mmol) of p...
Embodiment 3
[0061] Embodiment 3 ellipticine chromatographic analysis method
[0062] Ellipticine purity adopts HPLC method to measure, with ODS-3C18 chromatographic column, packing particle size 5μm, size 250×4.6mm, gradient elution with water (0.01% TFA)-methanol (0.01% TFA) as mobile phase (5%-95% methanol), flow rate 1.8ml / min, the column temperature is 45°C, the detection wavelength is 299nm, the elution time is 60min, and the purity of ellipticine calculated by area normalization is 95.75% ( Figure 7 and table below).
[0063] peak# keep time area high peak start peak end area% 1 27.52 7985137 545938 27.147 30.453 95.74627 2 43.187 126005 9103 42.901 43.616 1.456051 3 44.152 54325 2600 43.616 44.587 0.627764 4 45.241 173047 12995 45.003 48.32 1.999545 5 46.283 14746 1184 46.059 46.581 0.170368
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com