Method for synthesizing (R)-1,3-butanediol with whole cells of microorganism
A technology of butanediol and whole cells, applied in the field of asymmetric transformation of biocatalysis, can solve the problems such as the limited scale of the catalytic system of biochemical transformation efficiency, the inability to meet the requirements of industrialization, and the optical purity of the product not reaching 99%.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
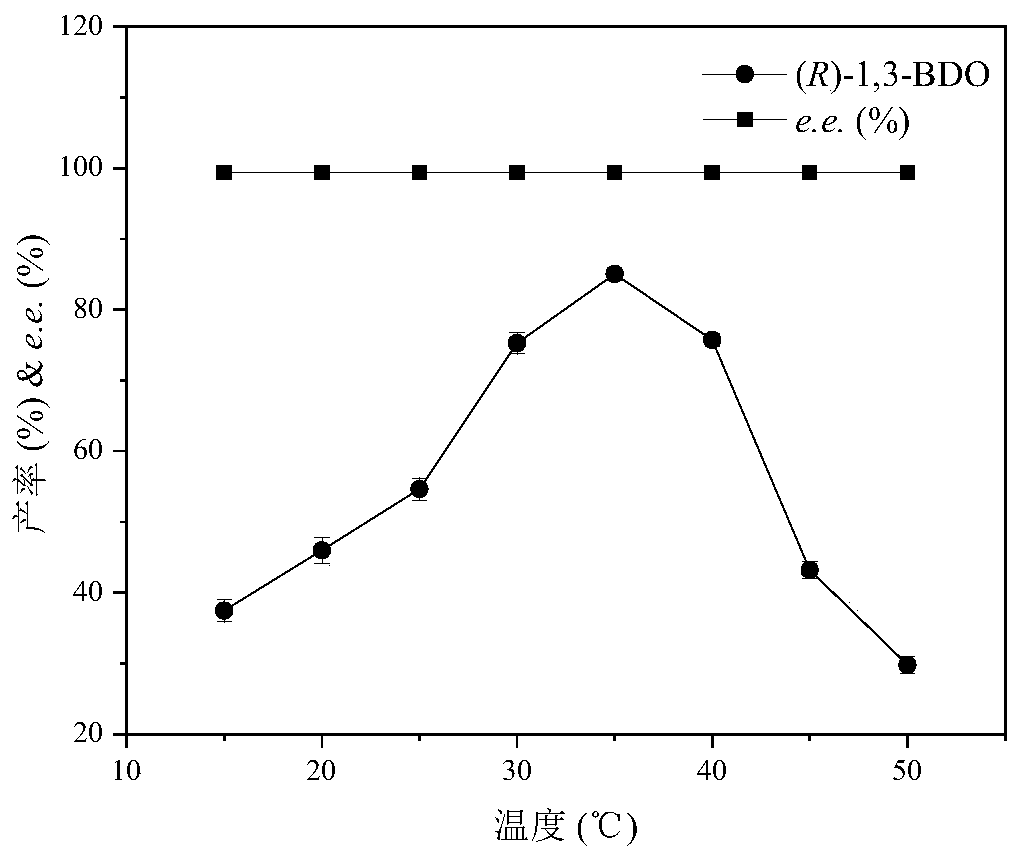
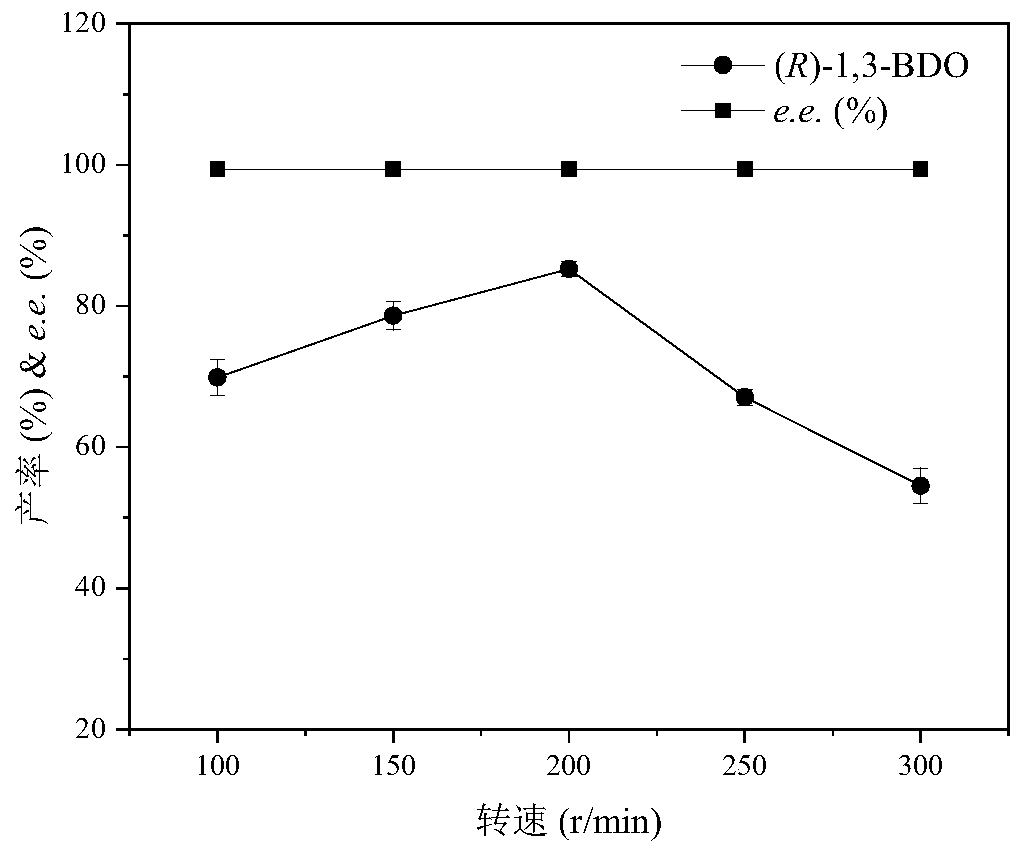
Image
Examples
Embodiment 1
[0032] The screening and identification of embodiment 1 highly efficient catalytic bacterial strain
[0033] In the strain screening process, 4H2B was used as the substrate and glucose was used as the auxiliary substrate to isolate and screen more than 200 strains of bacteria from the soil. After the screened strains were cultured in a 48-well plate for 48 hours, the bacteria were collected by centrifugation and placed in a 48-well plate. Add substrate and auxiliary substrate, react for 48 hours, detect and analyze the product after reaction, and use high product optical purity and yield as selection criteria to obtain a strain with good catalytic efficiency and high specificity for the substrate 4H2B, Finally, genetic identification was carried out. After identification, the strain was Pichia kudriazvii, and it was sent to the preservation institution for preservation with the preservation number CCTCC M 2019329.
Embodiment 2
[0034] Example 2 Preparation of whole cells of Pichia kudriavzevii CCTCC M 2019329
[0035] Pick a single colony of Pichia kudriavzevii CCTCC M 2019329 strain and inoculate it into a test tube containing 5mL of YPD liquid medium, cultivate it at 30°C and 200rpm for 16h, and transfer it to a 500mL Erlenmeyer shaker flask (containing 100mL YPD liquid medium), 30°C, 200rpm shaking culture for 48h, the fermentation broth was collected, centrifuged at 6000rpm for 10min, and washed with physiological saline for 3 times to collect the bacteria, which was used as a catalyst for the asymmetric transformation reaction.
Embodiment 3
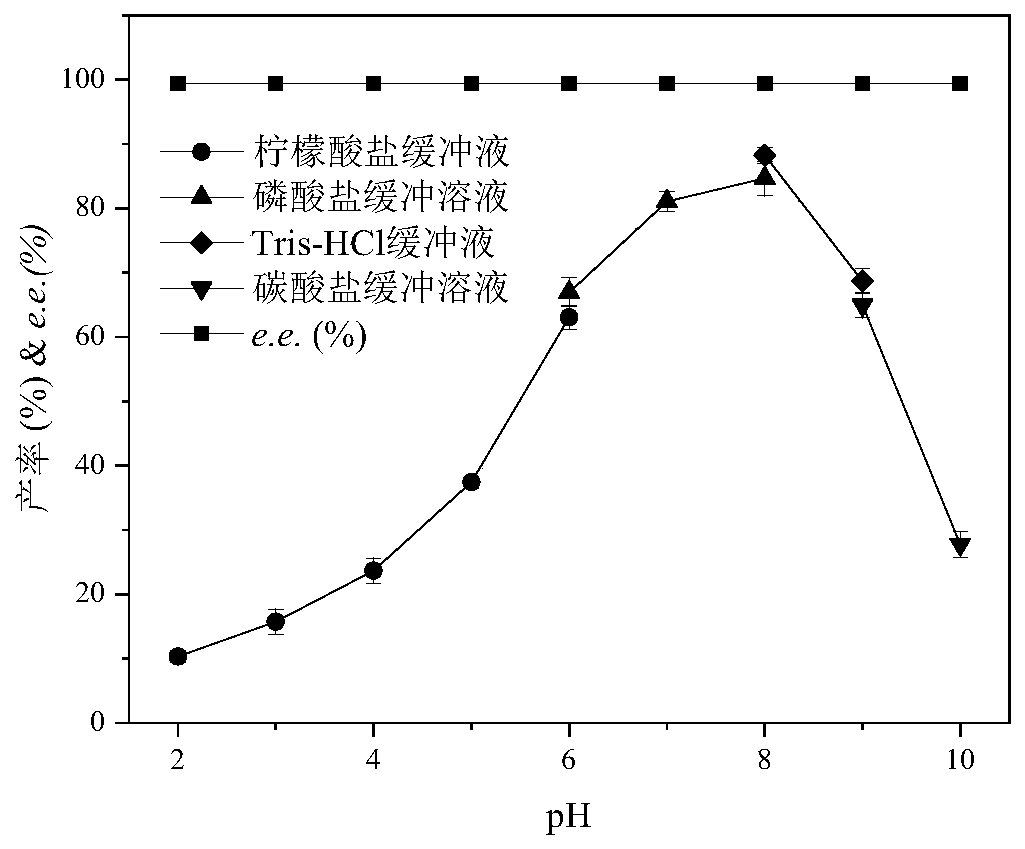
[0036] Example 3 Effects of different pH on the asymmetric reduction of 4H2B catalyzed by Pichia kudriavzevii CCTCC M 2019329
[0037] The catalytic reaction system is 10mL, the concentration of the substrate 4H2B is 20g / L, the final concentration of the catalyst is 1g / 10mL, the temperature is 30°C, the rotation speed is 200rpm, the auxiliary substrate is glucose, and the reaction is 12h.
[0038]Under different pH conditions, the catalytic activities of enzymes show great differences. By comparing the best buffers of different buffer solutions, the optimal pH (2.0-10.0) range is divided into 4 gradients: 0.1mol / L citrate buffer (pH 2.0-6.0), 0.1mol / L phosphate buffer (pH 6.0~8.0), 0.1mol / L Tris-HCl buffer solution (pH 8.0~9.0) and 0.1mol / L carbonate buffer solution (pH 9.0~10.0).
[0039] like figure 1 Shown, when the pH of reaction is lower than 5, the productive rate of product is lower than 40%; When the pH of reaction is greater than 8, along with the increase of pH, pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com