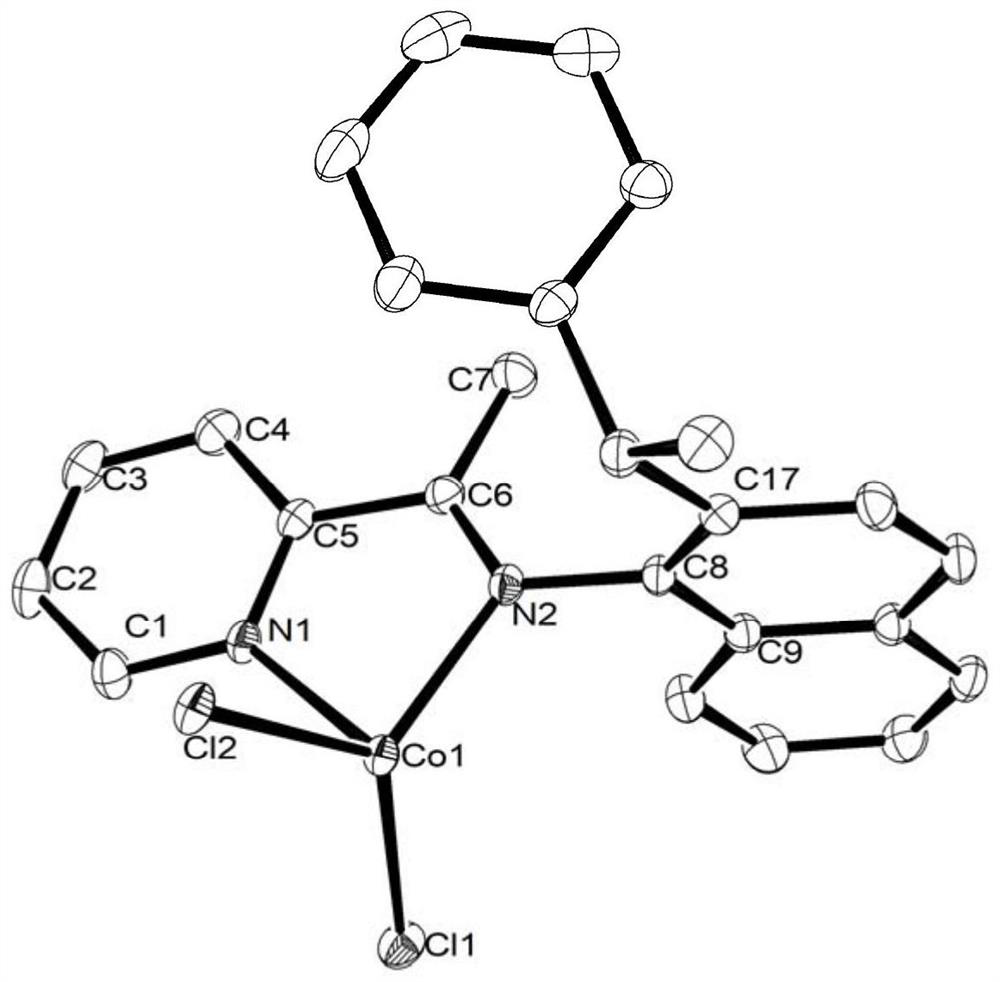
A kind of α-imine iron/cobalt complex catalyst and its preparation and use
A technology of catalysts and complexes, applied in the field of α-imine iron/cobalt complex catalysts and their preparation, to achieve stable performance, good catalytic activity, and simple operating conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
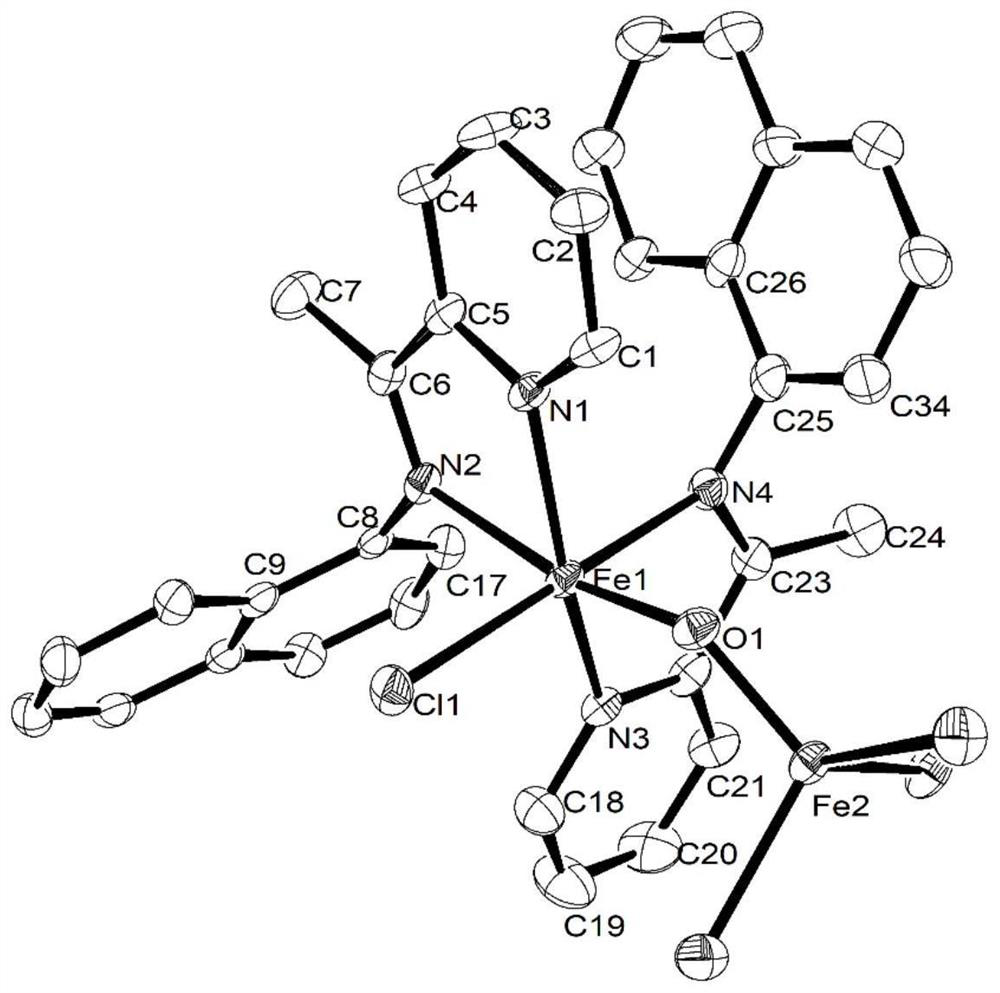
[0120] [2-(1-(1-naphthylamine) ethyl) pyridine] ferrous chloride [complex Fe1] shown in preparation formula Ⅰ-1:
[0121] At room temperature, the FeCl 2 4H 2 O (0.20g, 1.00mmol) and 2-(1-(1-naphthylamine)ethyl)pyridine (L1, 0.27g, 1.15mmol) were mixed and dissolved in ethanol, stirred for 12 hours under nitrogen protection, and removed under reduced pressure After ethanol was added to diethyl ether, a blue solid precipitated out, filtered, washed with diethyl ether, and dried to obtain a blue solid. Yield: 75%.
[0122] The structural confirmation data are as follows:
[0123] FT-IR (KBr, cm -1 ):3448(s), 3058(m), 2968(w), 1918(w), 2853(w), 1630(νC=N,s), 1595(s), 1573(w), 1508(m) ,1438(m),890(w),1369(s),1340(w),1262(m),1254(m),1227(w),1164(w),1126(w),1078(w) , 1016(w), 975(w), 870(w), 813(w), 766(s), 746(w), 735(w).
[0124] Elemental Analysis: C 34 h 28 Cl 2 FeN 4 (619.37) Theoretical value: C, 65.93, H, 4.56, N, 9.05. Experimental value C, 66.24, H, 4.90, N, 8.69...
Embodiment 2
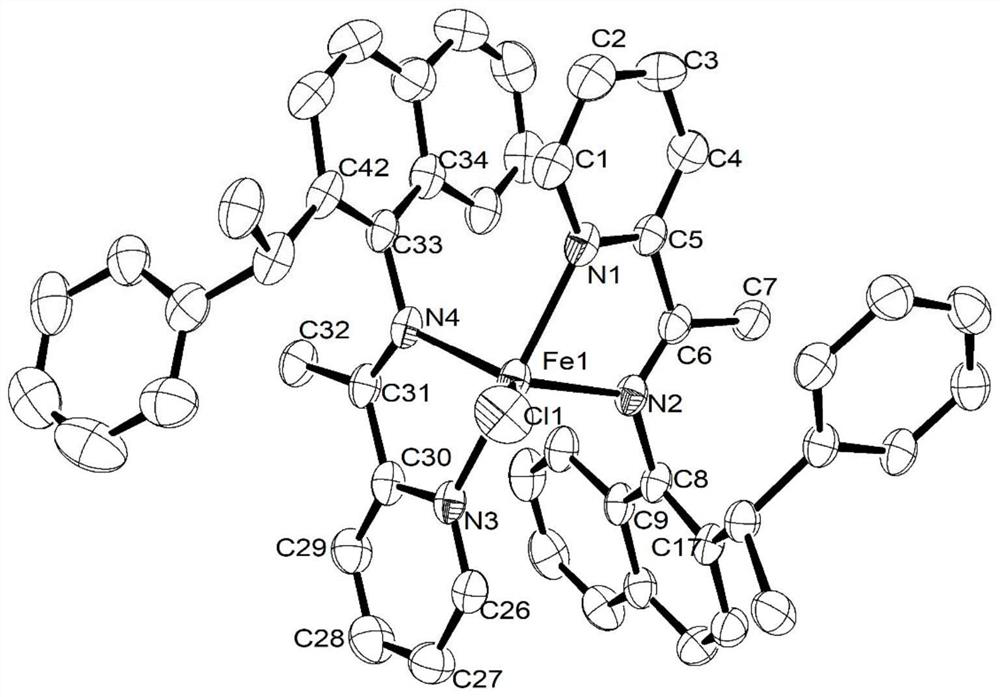
[0126] [2-(1-(2-methyl)-naphthylamine) ethyl) pyridine] ferrous chloride [complex Fe2] shown in preparation formula I-2:
[0127] At room temperature, the FeCl 2 4H 2 O (0.20g, 1.00mmol) and 2-(1-(2-methyl)-naphthylamine)ethyl)pyridine (L2, 0.30g, 1.15mmol) were mixed and dissolved in ethanol, and stirred for 12 hours under nitrogen protection After removing ethanol under reduced pressure, an orange solid was precipitated by adding ether, which was filtered, washed with ether, and dried to obtain an orange solid. Yield: 76%.
[0128] The structural confirmation data are as follows:
[0129] FT-IR (KBr, cm -1):3448(s), 3059(m), 2917(m), 1619(νC=N,s), 1592(s), 1570(w), 1507(w), 1440(m), 1372(s) ,1341(s),1316(w),1257(m),1234(w),1163(w),1129(w),1015(m),868(m),813(s),780(s) ,766(m),743(m).
[0130] Elemental Analysis: C 36 h 32 C l2 FeN 4 (647.43) Theoretical value: C, 66.79, H, 4.98, N, 8.65. Experimental value: C, 66.52, H, 4.78, N, 8.71.
Embodiment 3
[0132] [2-(1-(2-(1-phenylethyl)-naphthylamine) ethyl) pyridine] ferrous chloride [complex Fe3] shown in preparation formula I-3:
[0133] At room temperature, the FeCl 2 4H 2 O (0.02g, 0.10mmol) and 2-(1-(2-(1-phenylethyl)-naphthylamine)ethyl)pyridine (L3, 0.04g, 0.11mmol) were mixed and dissolved in ethanol, under nitrogen protection Stir under low pressure for 12 hours, remove ethanol under reduced pressure, add diethyl ether, a blue solid precipitates out, filter, wash with diethyl ether, and dry to obtain a blue solid. Yield: 65%.
[0134] The structural confirmation data are as follows:
[0135] FT-IR (KBr, cm -1 ):2970(s), 2905(s), 1620(νC=N,m), 1592(s), 1569(w), 1491(w), 1444(w), 1418(w), 1372(s) ,1310(s),1256(s),1158(w),1056(s),1021(w),974(w),903(w),866(s),830(m),809(w) ,772(m),746(s),702(s).
[0136] Elemental Analysis: C 50 h 44 C l2 FeN 4 (827.68) Theoretical value: C, 72.56, H, 5.36, N, 6.77. Experimental value: 73.00, H, 4.92, N, 6.72.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com