Anti-TIGIT antibodies and uses thereof
An antibody and antigen technology, applied in the direction of antibodies, applications, anti-infective drugs, etc., can solve the problems of no drugs, listing, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0637] Embodiment 1, preparation of anti-human TIGIT mouse monoclonal antibody
[0638] Human TIGIT ectodomain antigen (Uniport entry: Q495A1, produced by Anyuan Pharmaceutical Technology (Shanghai) Co., Ltd.) 50 μg was fully emulsified with complete Freund's adjuvant, and then immunized male Balb / C mice by multipoint immunization. The immunization cycle was Once every three weeks. On the 10th day after the third immunization, blood was collected from the tail vein, and the plasma anti-human TIGIT antibody titer was tested by ELISA to monitor the degree of immune response of the mice. Mice were boosted once. After 3 days, the mice were sacrificed and the spleens of the mice were taken out for fusion with the mouse myeloma Sp 2 / 0 cell line. Mix 2×10 8 Sp 2 / 0 cells with 2×10 8 Splenocytes were fused in 50% polyethylene glycol (molecular weight 1450) and 5% dimethyl sulfoxide (DMSO) solution. Adjust the number of spleen cells with HAT selection medium (DMEM medium containing...
Embodiment 2
[0639] Example 2, ELISA method to measure the binding ability of murine antibody and human TIGIT antigen
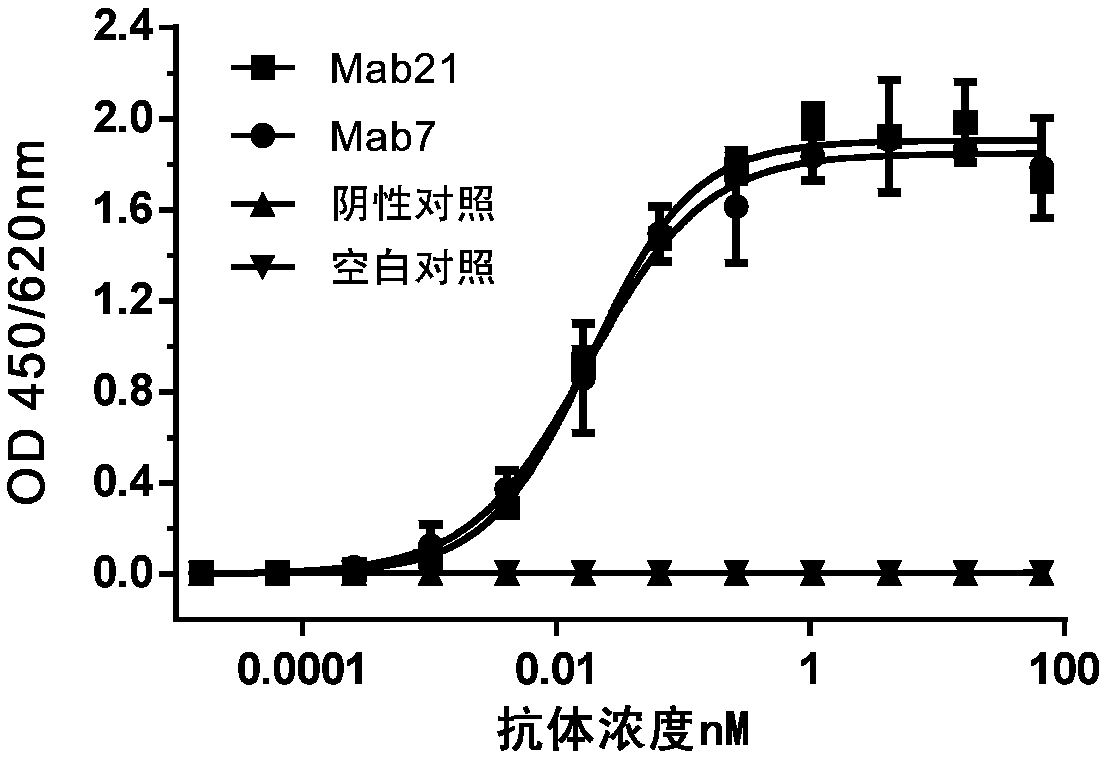
[0640] Coat the plate with human TIGIT and leave overnight at room temperature. The coating solution was discarded, the wells were blocked with skim milk dissolved in phosphate-buffered saline (PBS) for 1 hour, and the wells were washed with PBS containing 0.05% Tween-20. Then add 50 μL of purified anti-human TIGIT murine antibodies Mab21 and Mab7 to each well, use mouse IgG (prepared by purifying mouse serum with ProteinG) as a negative control, and PBS as a blank control, incubate at room temperature for 1 hour, and use 0.05% The wells were washed with Tween-20 PBS, and then 50 μL of HRP-labeled goat anti-mouse IgG polyclonal antibody (purchased from Jackson Laboratory, Cat. No.: 115-035-071) was added to each well as a detection antibody.
[0641] The result is as figure 1 As shown, the murine antibodies Mab21 and Mab7 have high affinity with human TIGIT, both EC 5...
Embodiment 3
[0642] Example 3, Subtype Identification and Variable Region Amplification of Anti-TIGIT Mouse Monoclonal Antibody
[0643] Antibody subtype identification: take hybridoma cell culture supernatant and use IsoStrip TM Mouse monoclonal antibody subtype identification kit (Santa Cruz Biotechnology, Cat. No. sc-24958) was used to identify antibody subtypes. The subtype of mouse monoclonal antibody Mab21 was identified as IgG2b (Kappa) type. The mouse monoclonal antibody Mab7 subtype was identified as IgG1 (Kappa) type.
[0644] Antibody variable region amplification: Candidate hybridoma cells #21 and #7 were cultured to a total of 10 7 Cells were collected by centrifugation at 1000 rpm for 10 min, and total RNA was extracted with an mRNA extraction kit (purchased from NEB Company, catalog number: S1550S), and the first Strand cDNA, using the first-strand cDNA as a subsequent template to amplify the DNA sequence of the antibody variable region corresponding to the hybridoma cell...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com