Medicine preparation containing anti-CD20 antibody as well as preparation method and application of medicine preparation
A preparation and antibody technology, which is applied to pharmaceutical preparations containing anti-CD20 antibodies and their preparation and application fields, can solve the problems of different environments and single types of humanized anti-CD20 antibody preparations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0204] 1. Preparation of anti-CD20 humanized monoclonal antibody
[0205] According to the humanized heavy chain variable region sequence (such as SEQ ID NO: 16 shown in Chinese patent application CN 201010150303.3) and light chain variable region sequence (such as the SEQ ID NO: 35 shown in Chinese patent application CN 201010150303.3) , design and synthesize PCR primer oligonucleotide fragments encoding heavy and light chain variable region sequences. Adjacent oligonucleotide fragments overlap by about 18 bases, and PCR primer oligonucleotide fragments generally have a length of about 54 bases. Mix equal amounts of each PCR primer fragment and perform overlap extension PCR reaction.
[0206] PCR reaction system: dNTPs 0.2 μM (final concentration); each PCR primer fragment 1 μl; 10×buffer 3 μl; clonedpfu (Invitrogen) 1 μl; add water to 30 μl.
[0207] PCR reaction conditions: 94°C for 3min→(94°C for 30s→56°C for 30s→72°C for 1min)×30→72°C for 10min.
[0208] The PCR produc...
Embodiment 1
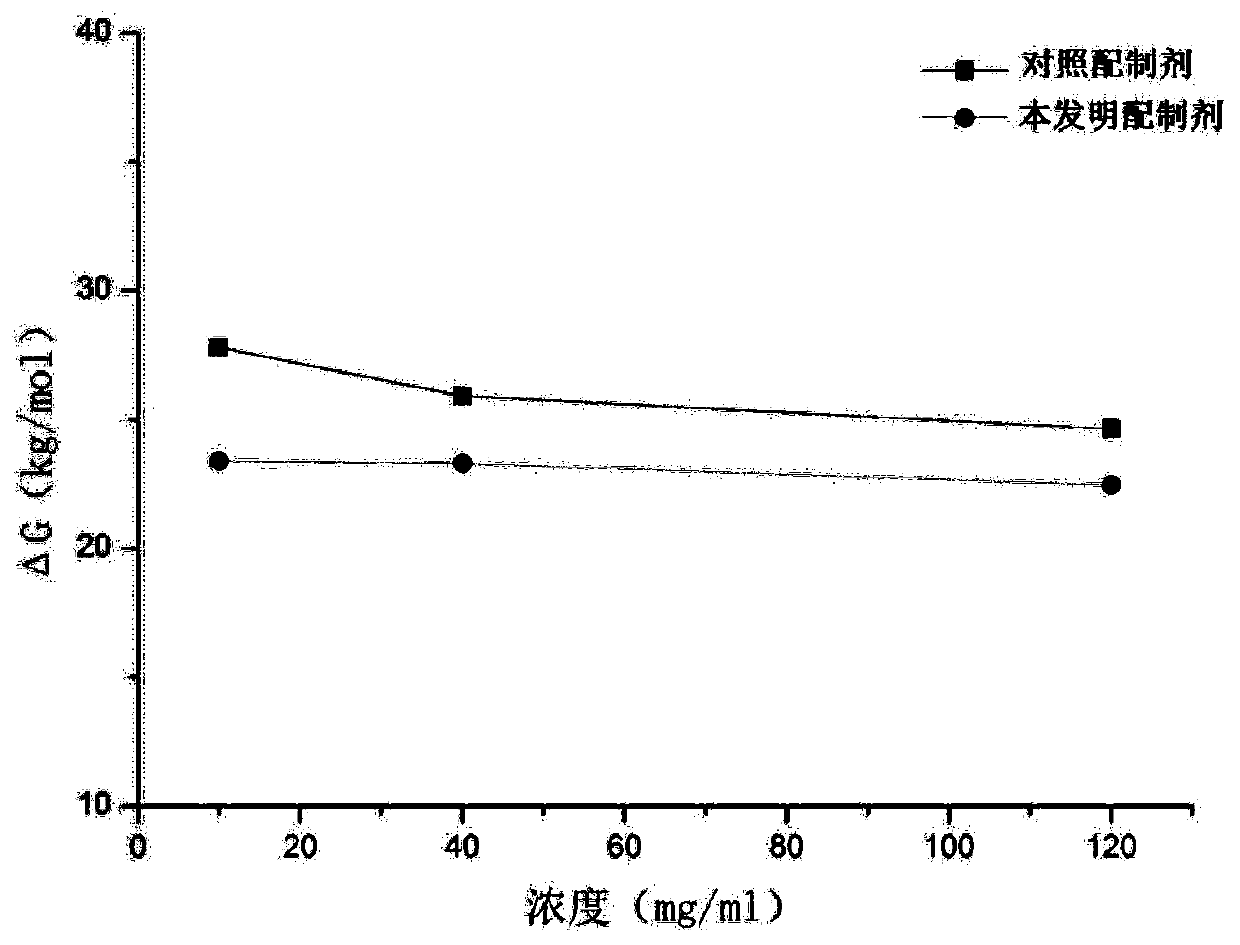
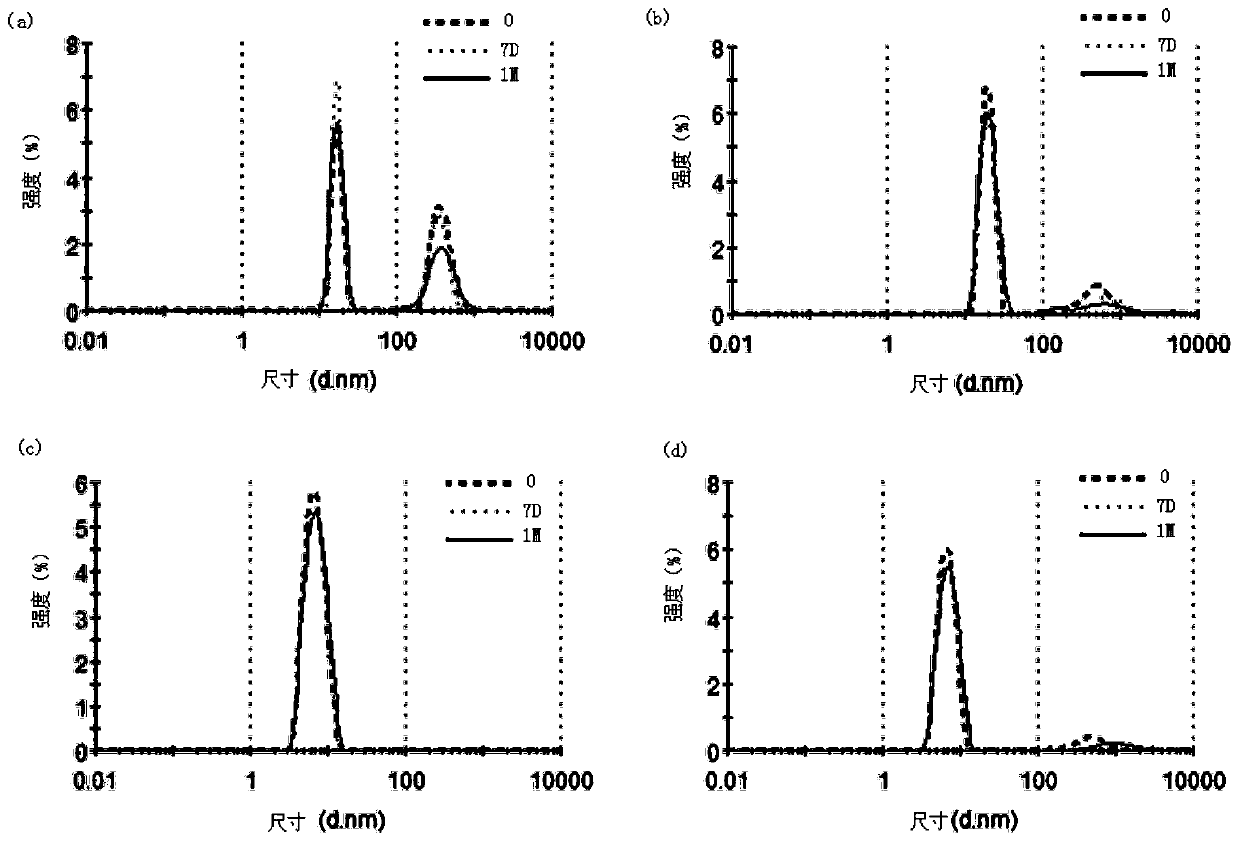
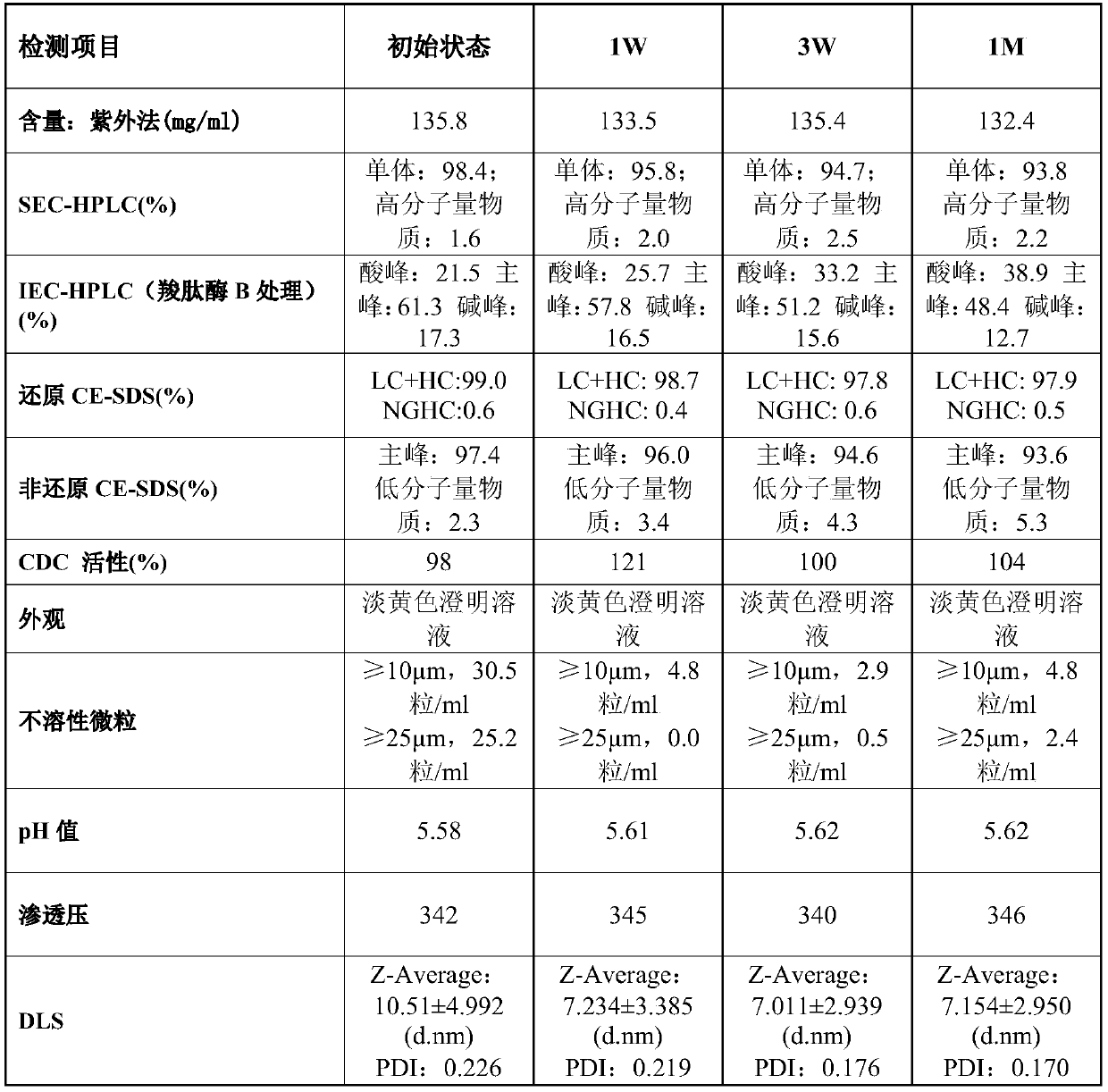
[0217] Example 1: Preparation of highly concentrated anti-CD20 antibody stock solution
[0218] To prepare liquid formulations, humanized anti-CD20 antibodies were buffer exchanged against diafiltration buffer containing the expected buffer composition and, where necessary, concentrated by diafiltration to an antibody concentration of approximately 130 mg / ml. After the diafiltration operation was completed, α,α-trehalose dihydrate, hyaluronidase produced by Suzhou Kangju and polysorbate 80 were added to the antibody solution as stock solutions. Finally, the protein concentration was adjusted to about 120 mg / ml with buffer.
[0219] Table 1
[0220] components concentration anti-CD20 antibody 120mg / mL L-histidine 3.4mM L-Histidine hydrochloride-hydrate 16.6mM L-methionine 10mM Tween 80 (Polysorbate 80) 0.06% α,α-Trehalose dihydrate 210mM
[0221] All formulations were sterile filtered through a 0.22 μm low protein bindi...
Embodiment 2
[0245] Example 2: Preparation of Humanized Anti-CD20 Liquid Formulation
[0246] To prepare the liquid formulation, the recombinant humanized anti-CD20 antibody (as disclosed in Patent No. ZL201010150303.3) was buffer-exchanged against a diafiltration buffer containing the expected buffer composition, and where necessary, Concentrate to an antibody concentration of approximately 150 mg / ml. After reaching the target concentration, α,α-trehalose dihydrate, hyaluronidase produced by Suzhou Kangju and polysorbate 80 were then added to the antibody solution as stock solutions. Finally, the protein concentration was adjusted to a concentration of approximately 120 mg / ml with the final formulation buffer.
[0247] Table 4
[0248] components concentration anti-CD20 antibody 120mg / mL L-histidine 3.4mM L-Histidine hydrochloride-hydrate 16.6mM L-methionine 10mM Tween 80 (Polysorbate 80) 0.06% α,α-Trehalose dihydrate 210mM hyalu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com