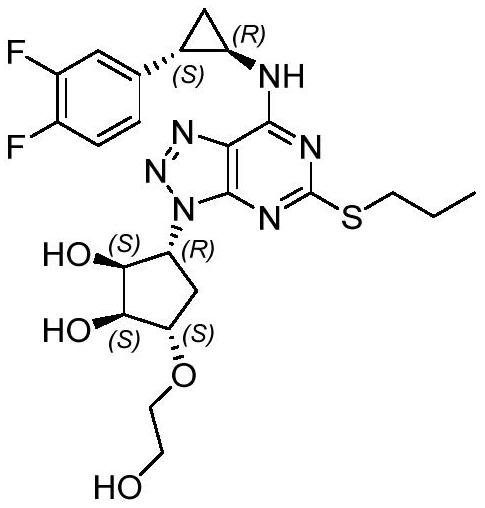
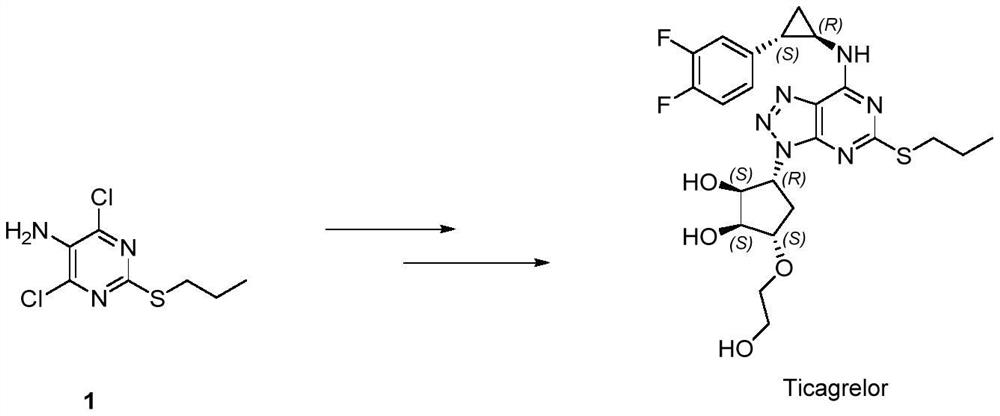
A kind of synthetic method of ticagrelor intermediate
A synthetic method, the technology of ticagrelor, which is applied in the field of synthesis of ticagrelor intermediates, can solve problems such as easy removal, limited stability of protecting groups, unfavorable quality of cyclization products, etc., and achieve high conversion rate and high reaction rate. Effects of mild conditions and cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
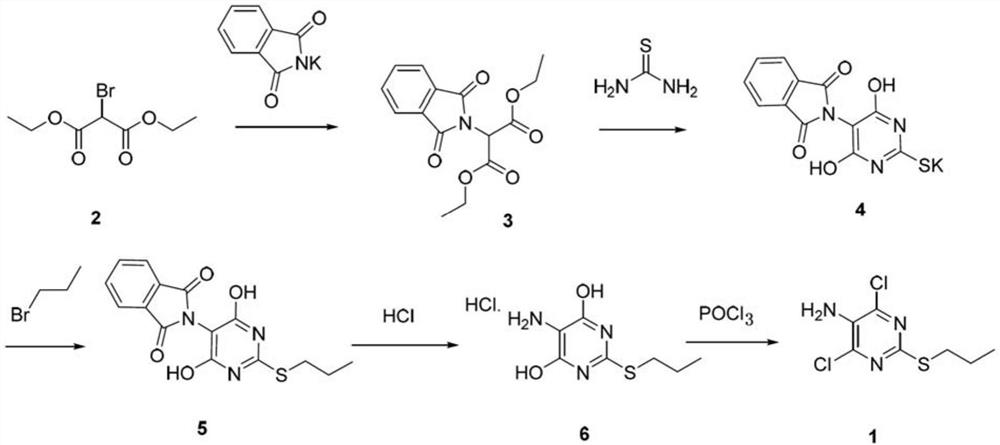
[0044] Example 1: Synthesis of compound 3 (diethyl 2-phthalimido-1,3-malonate)
[0045]
[0046] In the four-necked flask, add potassium phthalimide 22.2g, compound 2 (2-bromo-1,3-diethyl malonate) 24g, tetrabutylammonium bromide 1.2g, toluene 240mL, open Stir, and control the temperature to 20-25 ℃. The reaction was completed after 10 hours of incubation. Filter, rinse the filter cake with 25 mL of toluene once, add 120 mL of water to the filtrate, stir, let stand, and separate. The organic layer was washed once with 120 mL of water, the aqueous layer was extracted once with 120 mL of toluene, the organic layers were combined, dried with Yuanming Powder, filtered, the filter cake was rinsed once with 120 mL of toluene, and the filtrate was desolvated under reduced pressure to dryness to obtain 29.9 g of compound 3. The yield is 98%. ESI-HRMS(m / z):C 15 H 16 NO 6 [M+H + ] Theoretical calculated value: 306.0972, measured value: 306.0966. 1 H-NMR (400MHz, CDCl3) δ: 7.9...
Embodiment 2
[0047] Example 2: Synthesis of Compound 4 (Potassium 5-phthalimido-2-thio-barbiturate)
[0048]
[0049] Add 5 g of compound 3, 1.37 g of thiourea, and 30 mL of tert-butanol into a four-neck flask, stir, and slowly heat up to 80-85°C. Slowly add potassium tert-butoxide-tert-butanol solution (10 mL of tert-butanol, 3.85 g of potassium tert-butoxide) dropwise for 15-30 min. After the dropwise addition was completed, the reaction was completed in 6 hours. The temperature was slowly lowered to 25-30°C, filtered, the filter cake was rinsed once with 30 mL of ethyl acetate, put into a vacuum drying oven, and dried at a temperature of 50-55°C to obtain 5 g of compound 4 with a yield of 93%. ESI-HRMS(m / z):C 12 H 6 N 3 O 4 S[M-H + ] Theoretical calculated value: 288.0084, measured value: 288.0088. Compound 4 was directly used in the next step without further purification.
Embodiment 3
[0050] Example 3: Synthesis of compound 5 (5-phthalimido-2-propylthio-pyrimidine)
[0051]
[0052]Add 32.7 g of compound 4 (prepared according to the method of Example 2), 160 mL of water, and 160 mL of methanol into a four-necked flask, stir, and slowly heat up to 40-45°C. Slowly add 13.5g of 1-bromopropane dropwise, and keep stirring for 15min after the dropwise addition. 130 mL of NaOH (1 mol / L) was slowly added dropwise. After the dropwise addition was completed, the reaction was kept for 5 hours, and the reaction was complete. Slowly lower the temperature to 20-25°C, slowly add 30% hydrochloric acid dropwise, and adjust pH=2. The reaction was incubated for 1 hour, and then the temperature was slowly lowered to 0 to 5° C., and the reaction was incubated for 25 hours. After filtration, the filter cake was placed in a vacuum drying oven, and dried at a temperature of 50-55° C. to obtain 23.2 g of compound 5 with a yield of 70%. ESI-HRMS(m / z):C 15 H 14 N 3 O 4 S[M...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com