Method for generating 2-aminophenoloxazine-3-ketone compound by catalyzing oxidation of molecular oxygen in water phase
A technology of aminophenol oxazine and ketone compounds, applied in organic chemistry, etc., can solve the problems of low yield of target products in cross-coupling reactions, difficulty in avoiding salty wastewater, difficult post-treatment, etc., and achieve strong industrial application prospects , environmental friendliness, catalyst simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Synthesis of 2-aminophenoxazin-3-one:
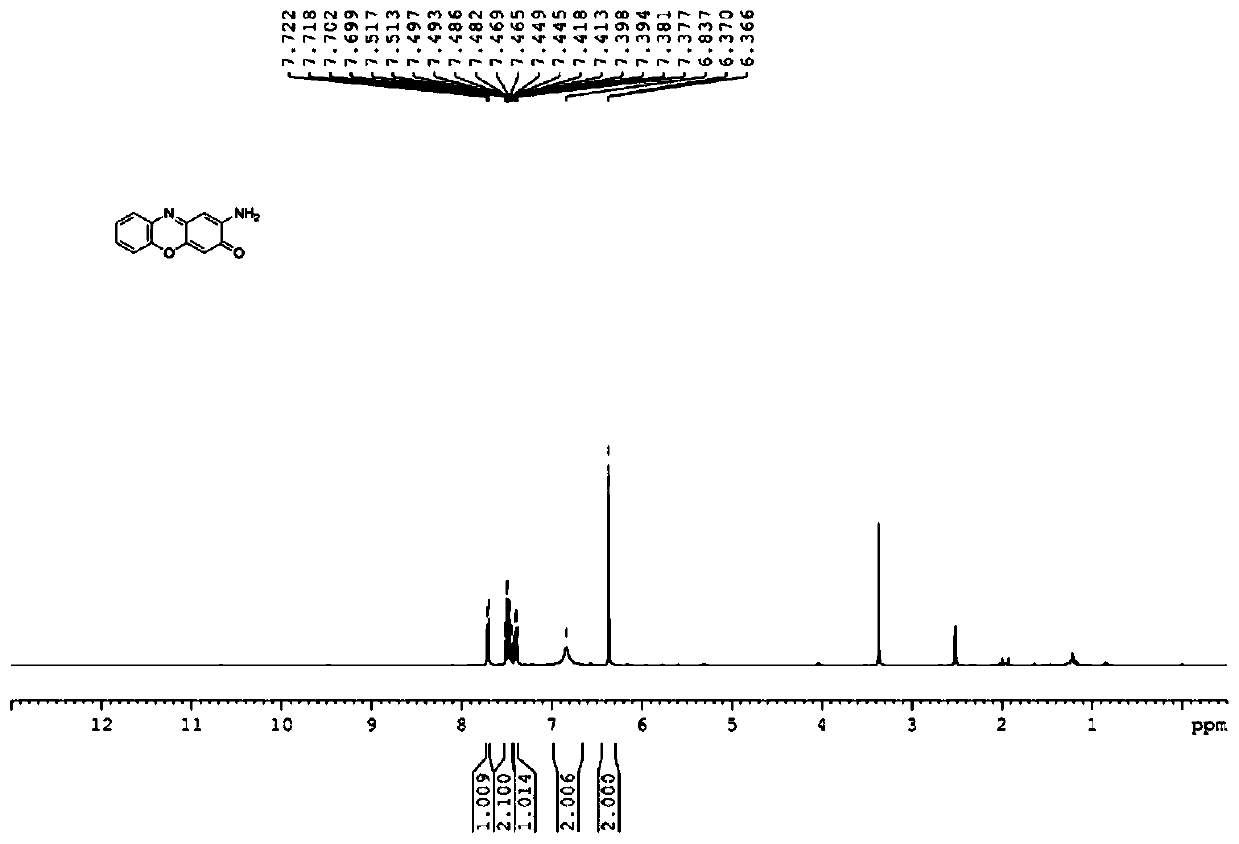
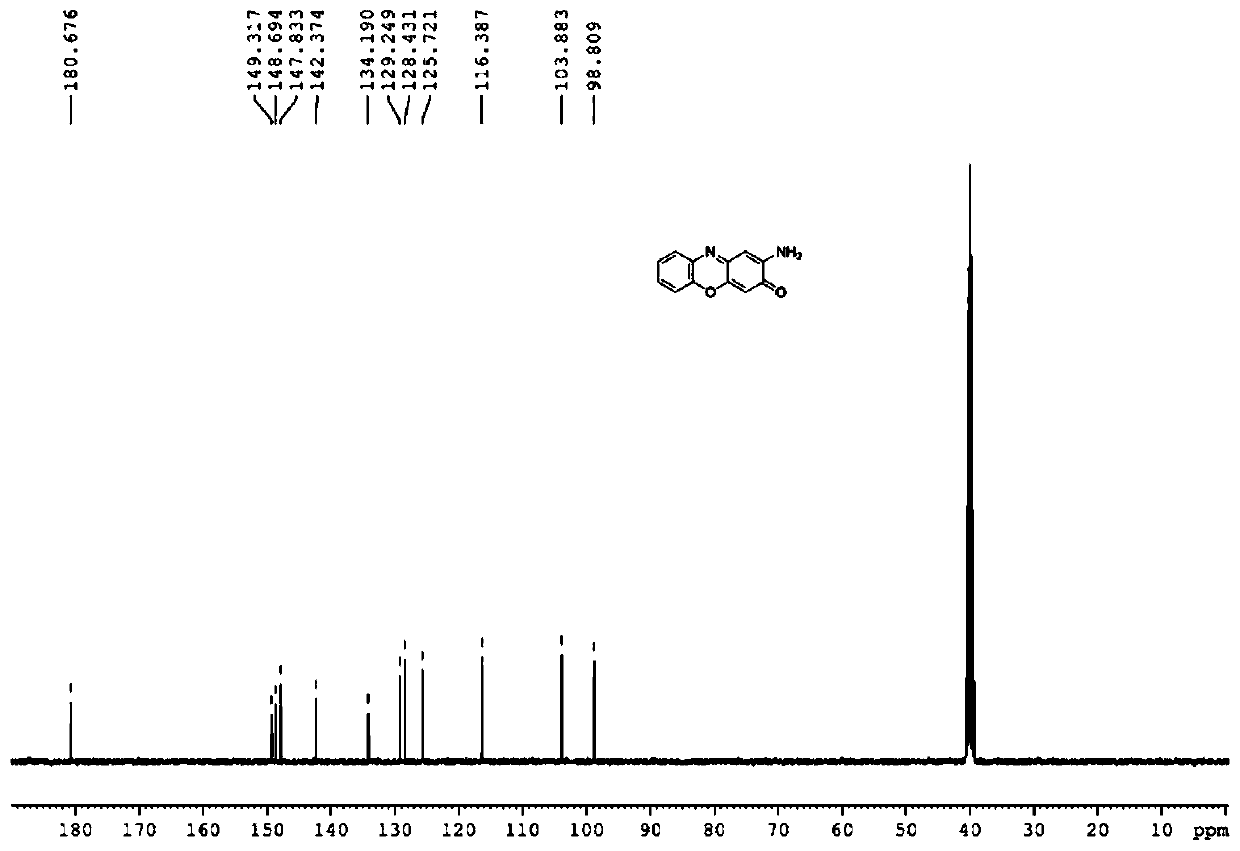
[0030] In a 150mL reactor, put 2.18g o-aminophenol, 218mg gallic acid, 2mg cobalt chloride, 13mg manganese sulfate, 138mg K 2 CO 3 and 80mL of water; heated to 15°C with stirring, introduced oxygen, kept the pressure in the reactor at 1.0MPa, stopped the reaction after 18 hours of reaction, cooled to room temperature, extracted with 3×15mL ethyl acetate, combined the ethyl acetate layers, Ethyl acetate was removed by rotary evaporation, and the remaining solid was recrystallized with isopropanol, suction filtered, and dried to obtain 2.04 g of a black solid. The product was passed through NMR (see attached figure 1 and 2 ), MS and other methods determine that the structure is 2-aminophenol oxazin-3-one, the yield is 94%, and the purity of the product analyzed by liquid chromatography is 97%.
Embodiment 2
[0032] Synthesis of 4-chloro-2-aminophenoxazin-3-one:
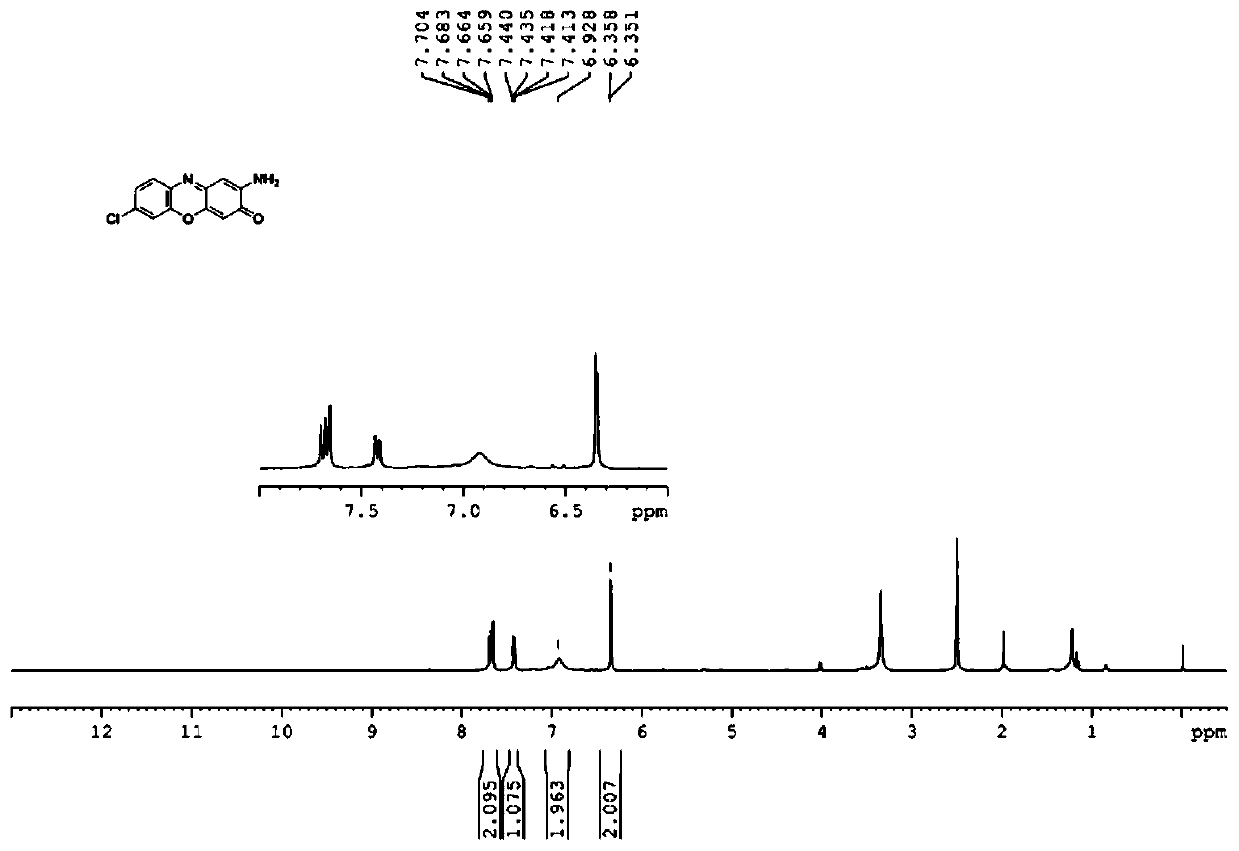
[0033] In a 150mL reactor, put 1.09g 2-aminophenol, 1.44g 4-chloro-2-aminophenol, 14mg gallic acid, 72mg copper chloride, 0.67g KOH and 65mL water; Air, keep the pressure in the reactor at 0.1MPa, stop the reaction after 4 hours of reaction, cool to room temperature, extract with 3×15mL ethyl acetate, combine the ethyl acetate layer, rotary evaporate to remove ethyl acetate, and reweight the remaining solid with isopropanol Crystallization, suction filtration, oven dry, obtain black solid 2.2g, product is through NMR (see attached image 3 and 4 ), MS and other methods determined that the structure was 7-chloro-2-aminophenoxazin-3-one, the yield was 87%, and the purity of the product analyzed by liquid chromatography was 97%.
[0034] Other 2-aminophenoxazin-3-one compounds were synthesized in the same manner as in Example 1, and their various reaction conditions and reaction results are shown in Table 1.
[0035] Tabl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com