An anti-tumor tetravalent platinum complex with anti-drug resistance function and its preparation method
A technology of platinum complexes and tetravalent platinum, applied in the field of medicine, can solve problems such as toxic side effects, drug resistance, and limited clinical use, and achieve the effects of low toxicity, excellent cytotoxic activity, and excellent antitumor effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
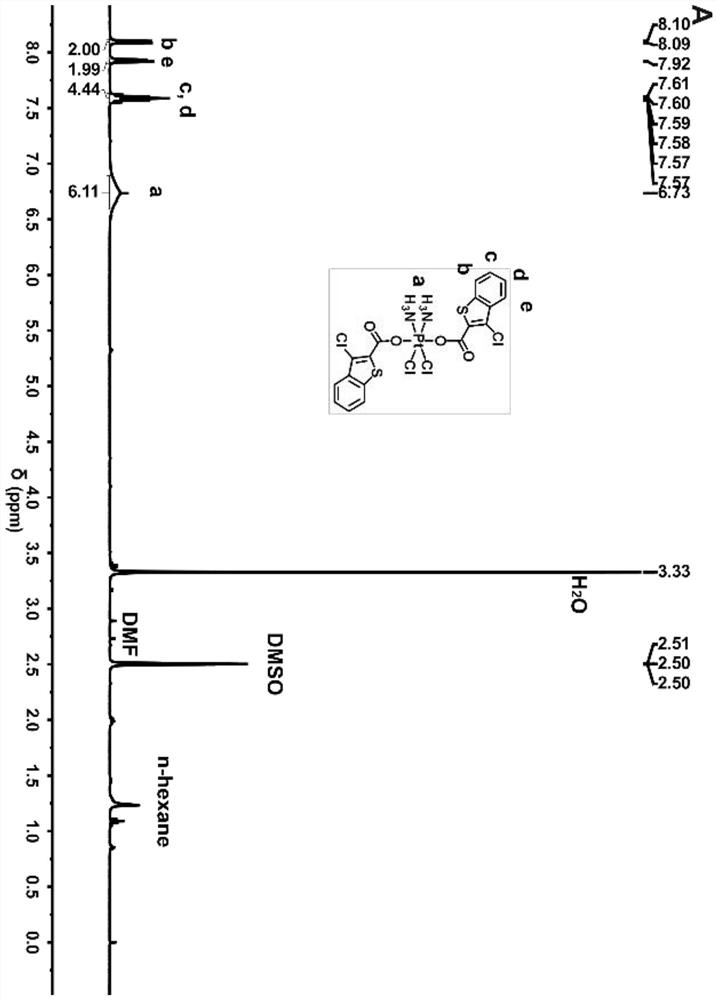
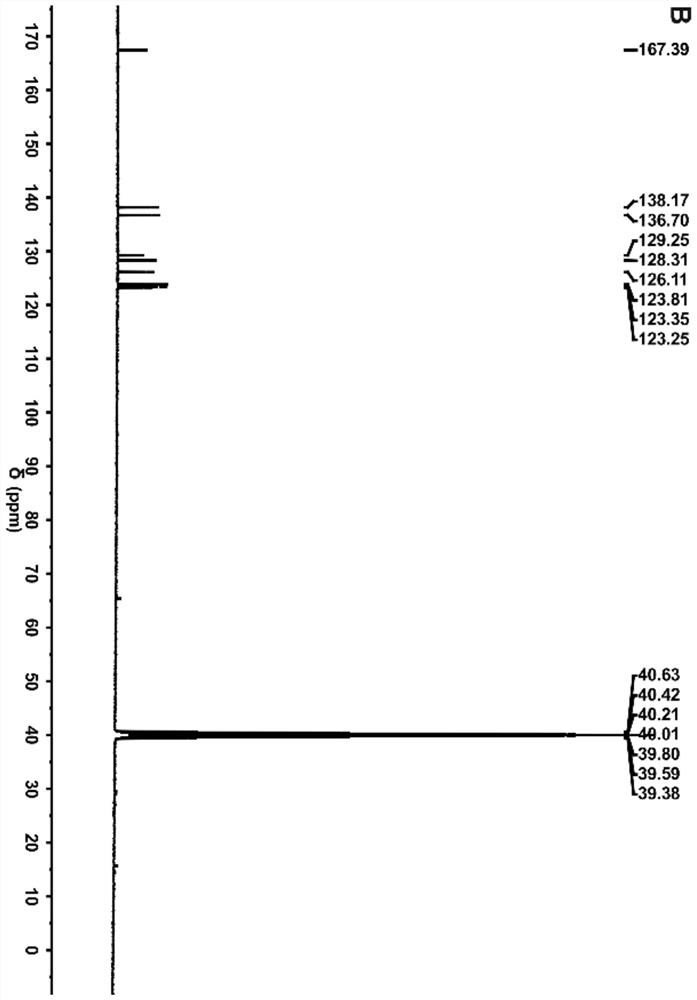
[0028] Synthesis of tetravalent platinum complexes 1 and 2
[0029]
[0030] S1. Put 1 g of cisplatin (Cisplatin) in a flask, then add 60 mL of 30% hydrogen peroxide dropwise, heat to 75°C and stir in the dark for 6 hours, then place it at room temperature for two days, and put it in a refrigerator at 4°C for two days, then filter and vacuum dry. Obtain tetravalent Oxoplatin yellow powder;
[0031] S2, take 100mg Oxoplatin, 191mg (or 204mg ), 125 μL of triethylamine (TEA) and 290 mg of O-benzotriazole-N,N,N',N'-tetramethyluronium tetrafluoroboric acid (TBTU) were dissolved in 10 mL of dry DMF at room temperature in the dark Stir for 48 hours;
[0032] S3, after the reaction is finished, remove the precipitate by filtration, concentrate the reaction solution under reduced pressure to 5 mL, add 20 mL of ethanol and water 1:1 mixture, settle out a light yellow solid, wash twice with methanol and ether respectively, to obtain a pure solid, The yield is about 80%.
Embodiment 2
[0033] The cytotoxic activity test of embodiment 2 compound 1 and 2
[0034] The compounds 1 and 2 prepared in Example 1 of the present invention were tested for their cytotoxic activity, using A549 / DDP, A549, A2780 / DDP, A2780, MCF-7, Caov3 and L-02 cells as models respectively, and synthesized in Example 1 Compounds 1, 2 and cisplatin are the substances to be detected. After the substances to be detected are applied to the cells, the survival of the cells is observed, and the cell viability is tested by the thiazolyl blue (MTT) method. The specific steps are as follows:
[0035] S1. Collect the above-mentioned logarithmic phase cells, adjust the concentration of the cell suspension, add them to a 96-well plate, and the number of cells per well is about 5000;
[0036] S2. Place the above-mentioned experimental cells in a cell incubator with a CO2 concentration of 5%, and culture them at 37° C. for 12 hours;
[0037] S3. Compounds 1, 2 and cisplatin were diluted with a medium ...
Embodiment 3
[0046] In vivo tumor inhibition test of embodiment 3 compound 1 and 2
[0047] After implanting human non-small cell lung cancer A549 cells subcutaneously into nude mice and forming tumors, the mice were divided into cages and grouped, with 5 mice in each group;
[0048] The method of tail vein injection is used to give mice administration, cisplatin 2mg / kg, compound 1 and 2 are both 4mg / kg, give once every three days and measure tumor volume and the body weight of mice, the result is as follows Figure 7 and 8 shown.
[0049]Compared with the traditional divalent platinum complex, the tetravalent platinum complex has two more ligands in the axial direction and forms an octahedral structure in space, so it is kinetically inert, has lower reactivity, and is less toxic. side effect. It is generally believed that tetravalent platinum complexes remain stable in plasma and normal tissues, and will be reduced to divalent platinum complexes in tumor hypoxia and highly reducing env...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com