Application of combination of chidamide and CGB as well as autologous hematopoietic stem cells and drug combination
A technology of hematopoietic stem cells and chidamide, which is applied in the application of chidamide combined with CGB and autologous hematopoietic stem cells and the field of combined drugs, can solve the problems of doubts about curative effect, poor curative effect, and inability of patients to get disease improvement, and achieve efficient treatment Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
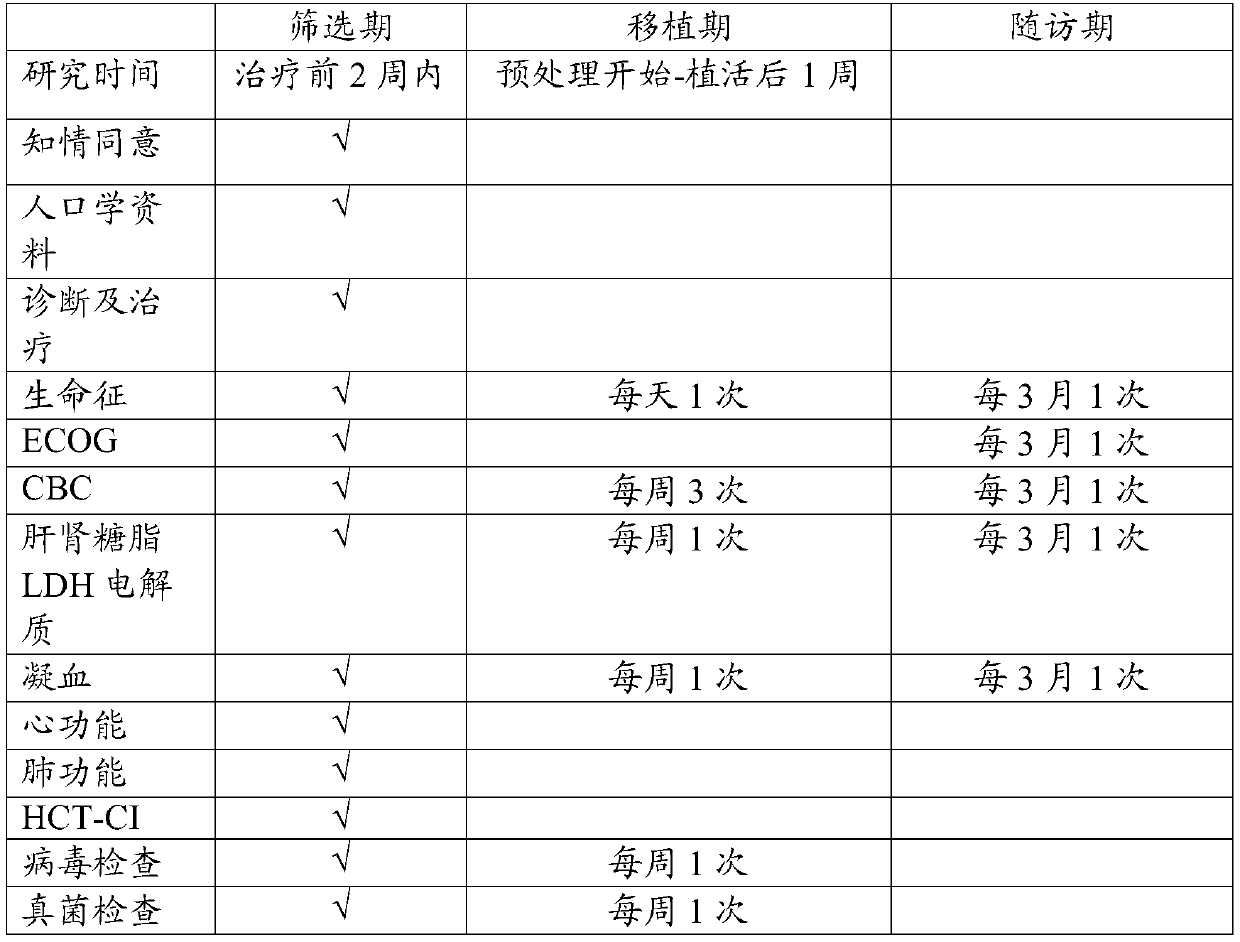
[0017] Example 1: Chidamide, cladribine, gemcitabine, and busulfan (ChiCGB) regimen combined with autologous hematopoietic stem cell transplantation in the treatment of relapsed or refractory diffuse large B-cell lymphoma in multicenter, single-arm, open-label II phase clinical trial
[0018] Test drug:
[0019] Chidamide tablets: off-white tablets, 5mg / tablet. Produced by Shenzhen Microchip Biotechnology Co., Ltd.
[0020] Cladribine: water injection, 10mg / bottle. Manufactured by Zhejiang Hisun Pharmaceutical Co., Ltd.
[0021] Gemcitabine: Use commercially available drugs.
[0022] Busulfan: water injection, 60mg / bottle. Produced by Otsuka Pharmaceutical Research and Development (Beijing) Co., Ltd.
[0023] Number of cases: A total of 93 patients are planned to be enrolled in this clinical trial.
[0024] Inclusion criteria: Patients must meet all of the following criteria to be enrolled.
[0025] 1. Diffuse large B-cell lymphoma who did not obtain CR in the first-li...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com