Ganglioside GM3 and/or analogue thereof, synthesis method and application of ganglioside GM3 and/or analogue thereof
A technology of gangliosides and synthetic methods, which is applied in the field of organic compound synthesis, and can solve problems such as undiscovered patent publications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0084] The embodiments of the present invention will be described in detail below. It should be noted that the embodiments are illustrative, not restrictive, and cannot limit the protection scope of the present invention.
[0085] The raw materials used in the present invention, unless otherwise specified, are conventional commercially available products; the methods used in the present invention, unless otherwise specified, are conventional methods in the art.
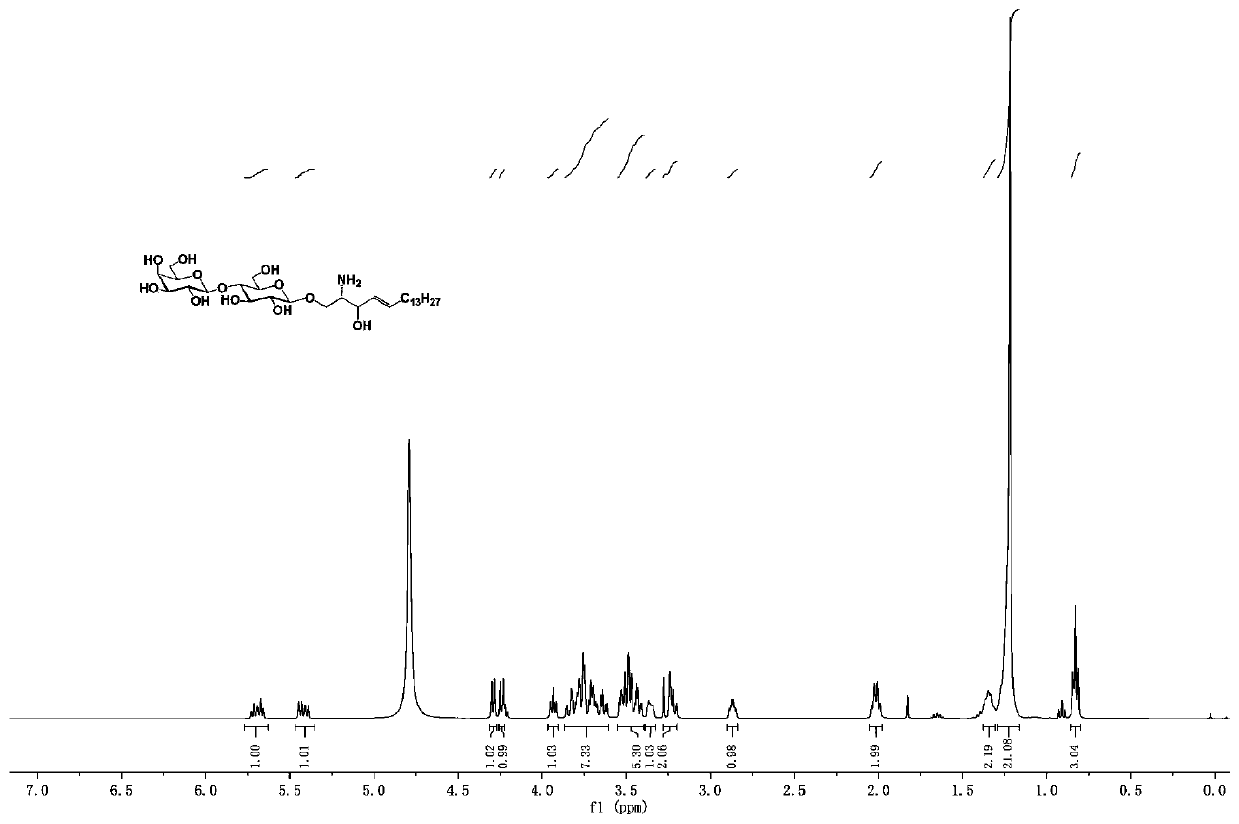
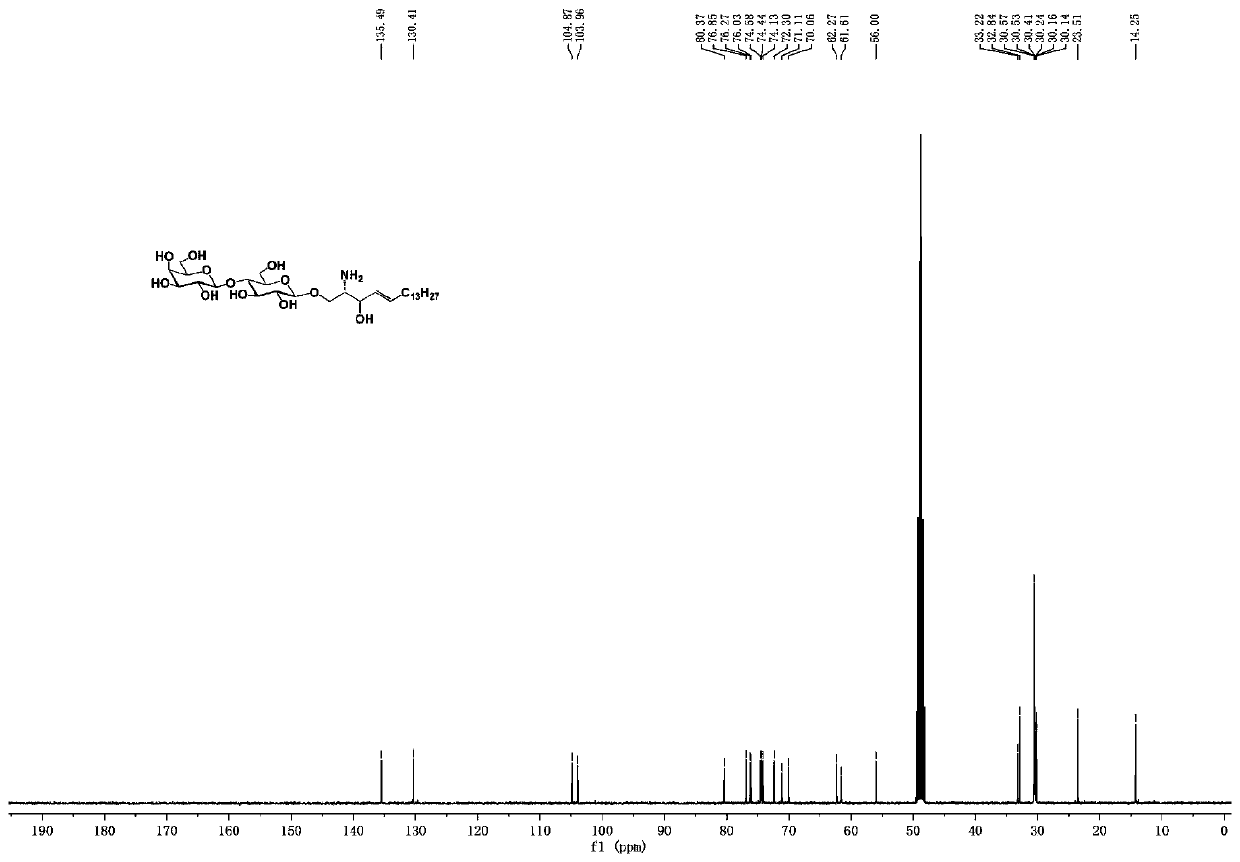
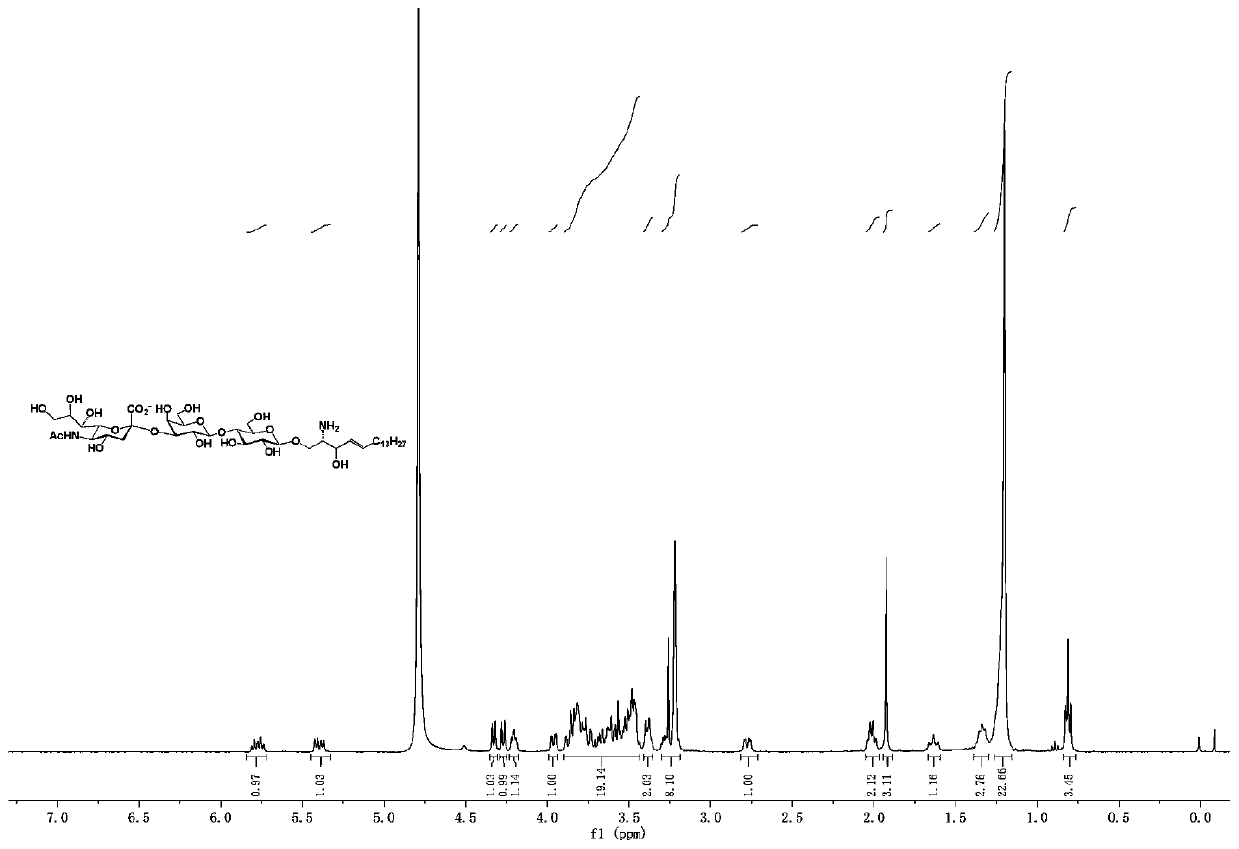
[0086] A ganglioside GM3 and / or its analogue, its structural formula is as follows:
[0087]
[0088] in:
[0089] R 2 , selected from fluorine atom, hydrogen atom, azide, hydroxyl, acetyl, trimethylacetyl;
[0090] R 3 , selected from fluorine atom, hydrogen atom, acetyl group, hydroxyl group;
[0091] R 4 , selected from fluorine atom, hydrogen atom, acetyl group, hydroxyl group;
[0092] R 7 , selected from nitrogen acetamide, nitrogen propionyl amino, nitrogen trifluoroacetamide, nitrogen azidoacetamide;...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com