Integrin-targetable polypeptide mn and application thereof in preparing tumor targeting drugs
An integrin and targeting technology, applied in the field of pharmacy, can solve problems such as insufficient stability, and achieve the effects of overcoming insufficient stability, improving binding activity, and improving tumor targeting and diagnosis and treatment in vivo
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
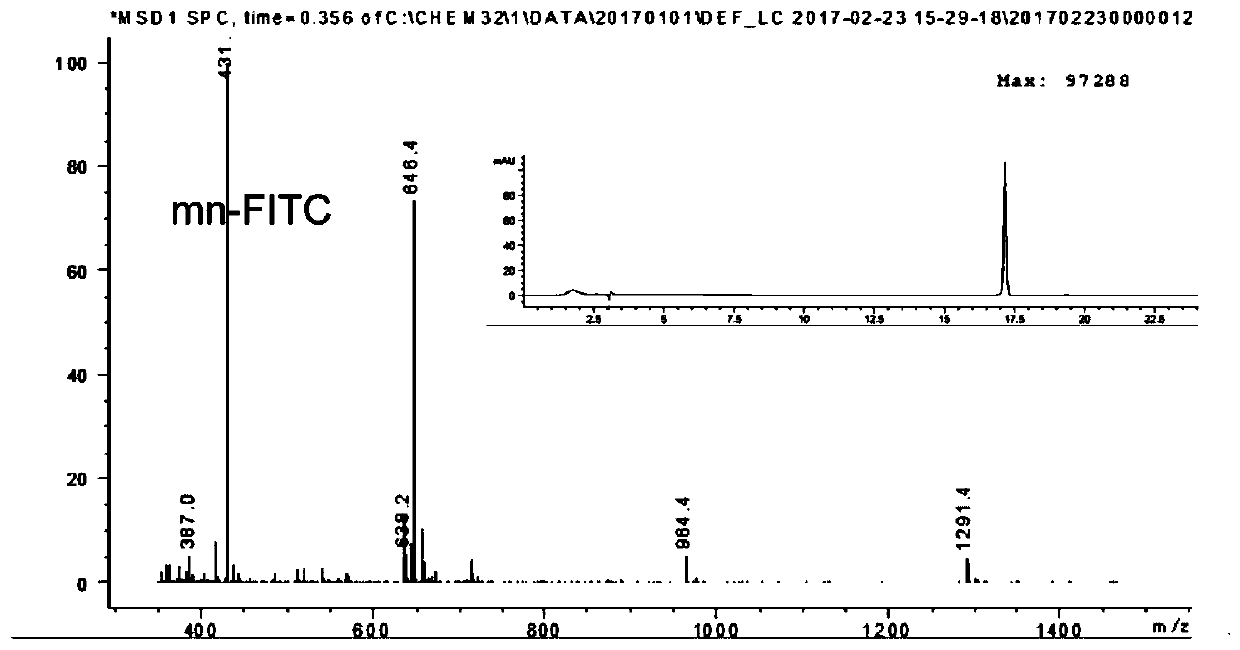
[0100] Synthesis and Characterization of Peptide, Peptide-Cys, Peptide-Fluorescein, Peptide-Drug Complex, Peptide-PEG-DSPE
[0101] Synthesis and Characterization of Peptides and Peptide-Cys:
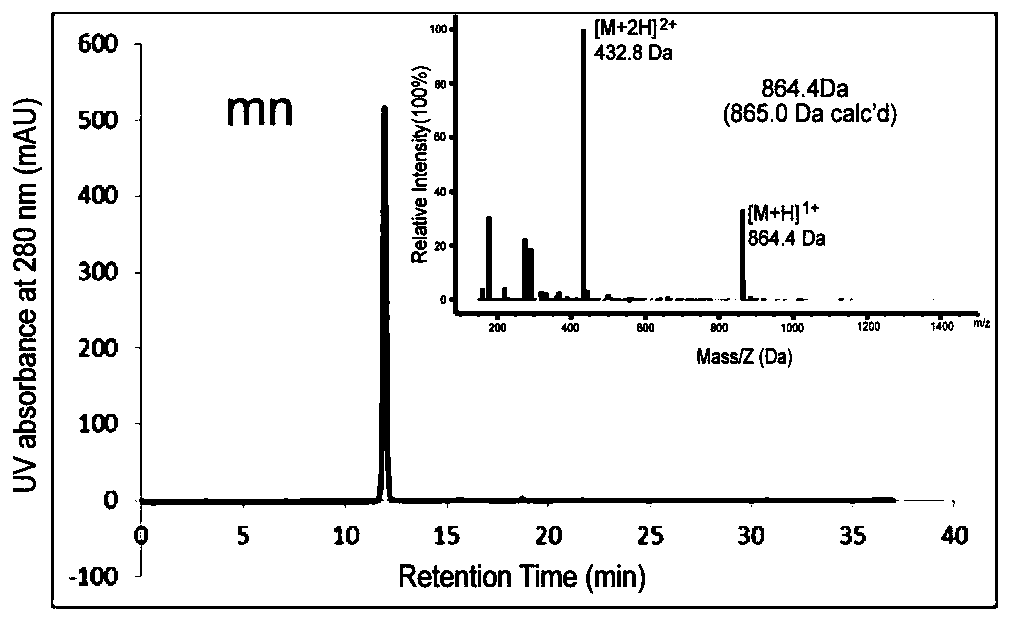
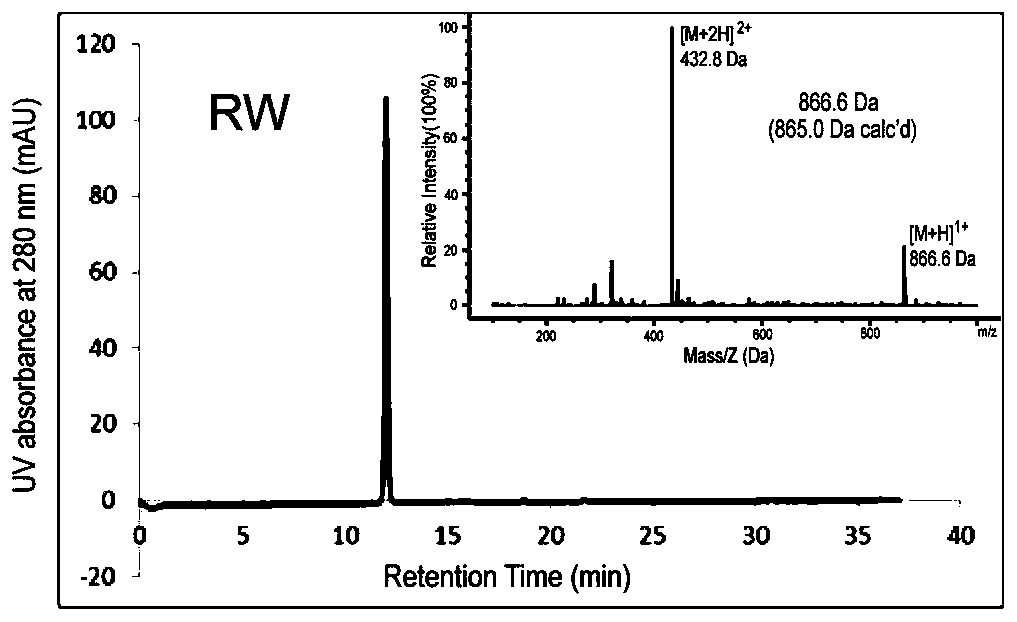
[0102]The solid-phase peptide synthesis method was used to design and synthesize the mn polypeptide (amino acid sequence mnRwr; uppercase letters indicate amino acids in L configuration, lowercase letters indicate amino acids in D configuration) and synthesize RW polypeptides (amino acid sequence RWrNM; uppercase letters indicate amino acids in L configuration, Lowercase letters indicate D-configuration amino acids).
[0103] Specific method: use Boc solid-phase peptide synthesis method, insert amino acids in sequence on PAM-Boc resin, and react with HBTU / DIEA as condensation agent and TFA as deprotection agent. After the reaction was completed, the resin was cut with hydrogen fluoride containing P-cresol, and stirred in an ice bath for 1 h. After the reaction, the hydrogen fluoride i...
Embodiment 2
[0114] Study on Serum Stability of Peptides
[0115] Make c(RGDyK), RW and mn into 1mg / mL aqueous solution, take 0.1mL and add it to 0.9mL 25% mouse serum, incubate at 37°C, at 0 and 15min, 0.5, 1, 2, 4, 8 and After 12 hours, 100 μL of the reaction solution was taken out, 20 μL of trichloroacetic acid (TCA) was added to precipitate proteins in the serum, left at 4°C for 20 min, centrifuged at 12,000 rpm for 10 min, and 20 μL of the supernatant was taken for HPLC analysis (eg Image 6 shown).
Embodiment 3
[0117] Polypeptide and Integrin α v beta 3 binding activity assay
[0118] The recombinant human GRP78 was coupled to the CM5 chip, and the RU value reached the target value. Configure c(RGDyK), RW and mn respectively as a gradient sample solution. Samples were injected sequentially from low to high, and the binding activities of c(RGDyK), RW and mn to proteins were analyzed with Biacore T200Evaluation software, and their K were calculated respectively D value (such as Figure 7 shown).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com