Neutralizing antibody B826 of hepatitis b virus and application of neutralizing antibody B826
A hepatitis B virus and antibody technology, applied in applications, antibodies, antiviral agents, etc., can solve problems such as large side effects, low cost-effective treatment effect, and inability to completely remove hepatitis B virus.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1, the discovery of antibody
[0036] Using a single B cell clone, looking for monoclonal antibodies to hepatitis B virus from the blood of hepatitis B vaccine immunized persons, and then comparing the effects of each antibody obtained.
[0037] Antigen bait flow sorting method for cloning hepatitis B small membrane protein-specific B cell antibody gene: isolate peripheral blood lymphocyte PBMC from the peripheral blood of normal persons immunized with hepatitis B vaccine, sort memory B cells with magnetic beads, and use flow cytometry The cytometer sorts out the cells specifically bound to the hepatitis B small membrane protein from the memory B cells, lyses the cells, and obtains cDNA by RT-PCR, and then performs nested PCR with specific primers for the variable region of the antibody, and sequence the antibody gene. Further screening confirmed that the antibody was hepatitis B small membrane protein monoclonal antibody.
[0038] A monoclonal antibody (bin...
Embodiment 2
[0040] Embodiment 2, the preparation of monoclonal antibody B826
[0041] 1. Preparation of recombinant plasmids
[0042] Insert the DNA molecule shown in Sequence 2 of the sequence listing into pLB-simple Vector to obtain a recombinant plasmid. The recombinant plasmid has been verified by sequencing. This plasmid is also named heavy chain expression plasmid.
[0043] In Sequence 2 of the sequence listing, nucleotides 1-666 are the CMV promoter, nucleotides 915-971 are the signal peptide coding region, and nucleotides 972-1340 are the heavy chain variable region coding region 1341-2330 nucleotides are the coding region of the heavy chain constant region, 2331-2333 are stop codons, and 2392-2540 nucleotides are polyA terminators. The DNA molecule shown in Sequence 2 of the Sequence Listing expresses the heavy chain shown in Sequence 1 of the Sequence Listing.
[0044] Insert the DNA molecule shown in Sequence 4 of the sequence listing into pLB-simple Vector to obtain a reco...
Embodiment 3
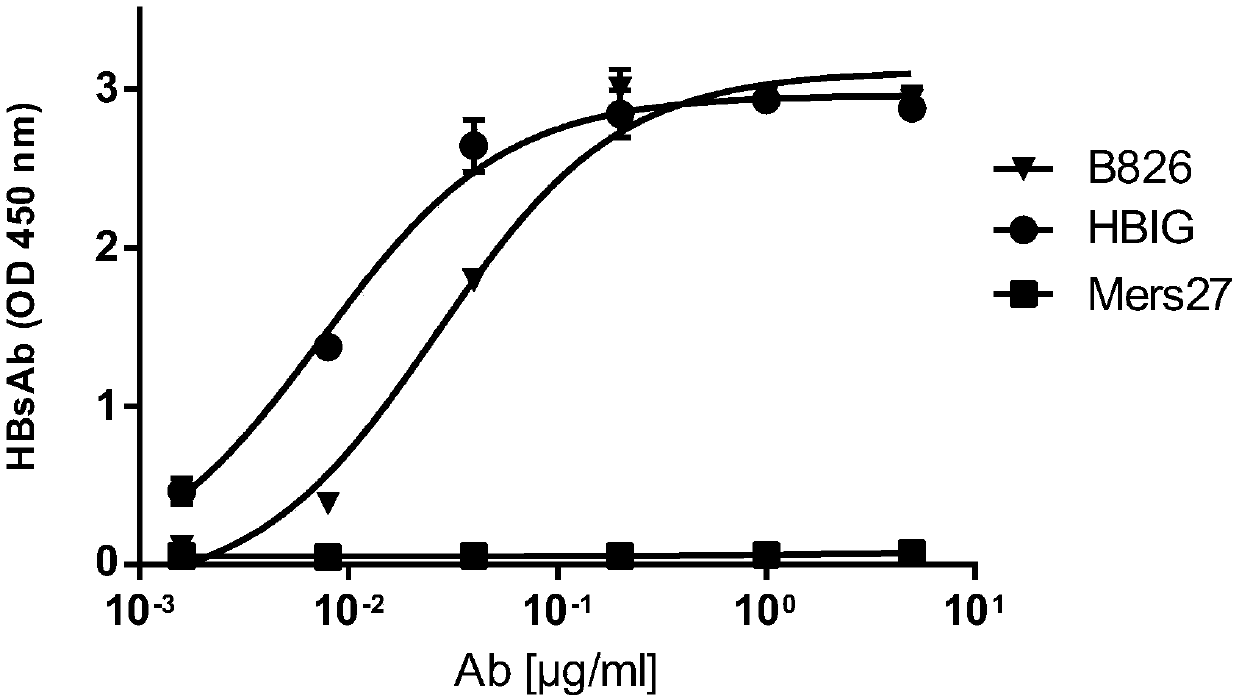
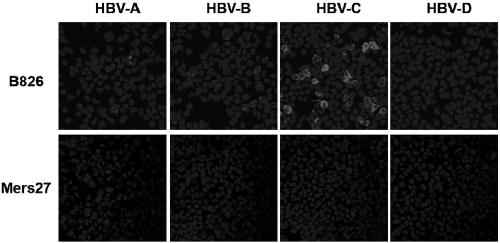
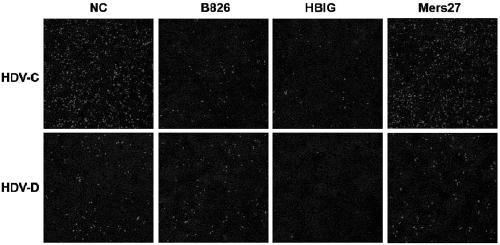
[0055] Embodiment 3, the binding ability of antibody and hepatitis B virus
[0056] 1. ELISA detection
[0057] 1. Take the ELISA plate and add the original coating solution for coating (50ng original coating / well).
[0058] Coating was originally HBs antigen. A coating stock solution was prepared using PBS buffer as a solvent.
[0059] 2. After completing step 1, take the ELISA plate, add 200 μl of PBS buffer solution containing 2% BSA to each well, incubate at 37°C for 2 hours to block, then discard the supernatant and wash twice with PBST solution.
[0060] 3. After completing step 2, take the ELISA plate, add 200 μl of antibody diluent to each well, incubate at 37°C for 1 hour, then discard the supernatant and wash twice with PBST solution. The antibody diluent is obtained by diluting the B826 antibody solution or the HBIG solution or the MERS-27 antibody solution prepared in Example 2 with PBS buffer. Five replicate wells were set up for each antibody dilution.
[00...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap