Neutralizing antibody B430 of hepatitis B virus (HBV) and application of neutralizing antibody B430
A hepatitis B virus and antibody technology, applied in applications, antibodies, antiviral agents, etc., can solve problems such as difficulty in reducing hepatitis B surface antigen, long interferon treatment cycle, and large side effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1, the discovery of antibody
[0035] Using the culture of human memory B cells, looking for monoclonal antibodies to hepatitis B virus from the blood of hepatitis B vaccine immunized persons, and then comparing the effects of each antibody obtained.
[0036] B cell culture screening method for cloning hepatitis B small membrane protein-specific B cell antibody gene: isolate peripheral blood lymphocyte PBMC from the peripheral blood of normal people immunized with hepatitis B vaccine, and sort out memory B cells from PBMC by flow cytometry. Co-culture memory B cells and supporting cells 3T3-CD40L for 10 days, use Elisa to detect whether the secreted cell supernatant contains hepatitis B virus small membrane protein HBs antibody, antibody-positive holes are lysed to reverse transcribe cDNA from B cells, and use variable antibody Region-specific primers were used to clone all antibody variable region genes of positive wells, and a pair of hepatitis B small memb...
Embodiment 2
[0039] Embodiment 2, the preparation of monoclonal antibody B430
[0040] 1. Preparation of recombinant plasmids
[0041] Insert the DNA molecule shown in Sequence 2 of the sequence listing into pLB-simple Vector to obtain a recombinant plasmid. The recombinant plasmid has been verified by sequencing. This plasmid is also named heavy chain expression plasmid.
[0042] In Sequence 2 of the sequence listing, nucleotides 1-666 are the CMV promoter, nucleotides 915-971 are the signal peptide coding region, and nucleotides 972-1343 are the heavy chain variable region coding region , 1344-2333 nucleotides are the heavy chain constant region coding region, 2334-2336 are stop codons, and 2395-2543 nucleotides are polyA terminators. The DNA molecule shown in Sequence 2 of the Sequence Listing expresses the heavy chain shown in Sequence 1 of the Sequence Listing.
[0043] Insert the DNA molecule shown in Sequence 4 of the sequence listing into pLB-simple Vector to obtain a recombina...
Embodiment 3
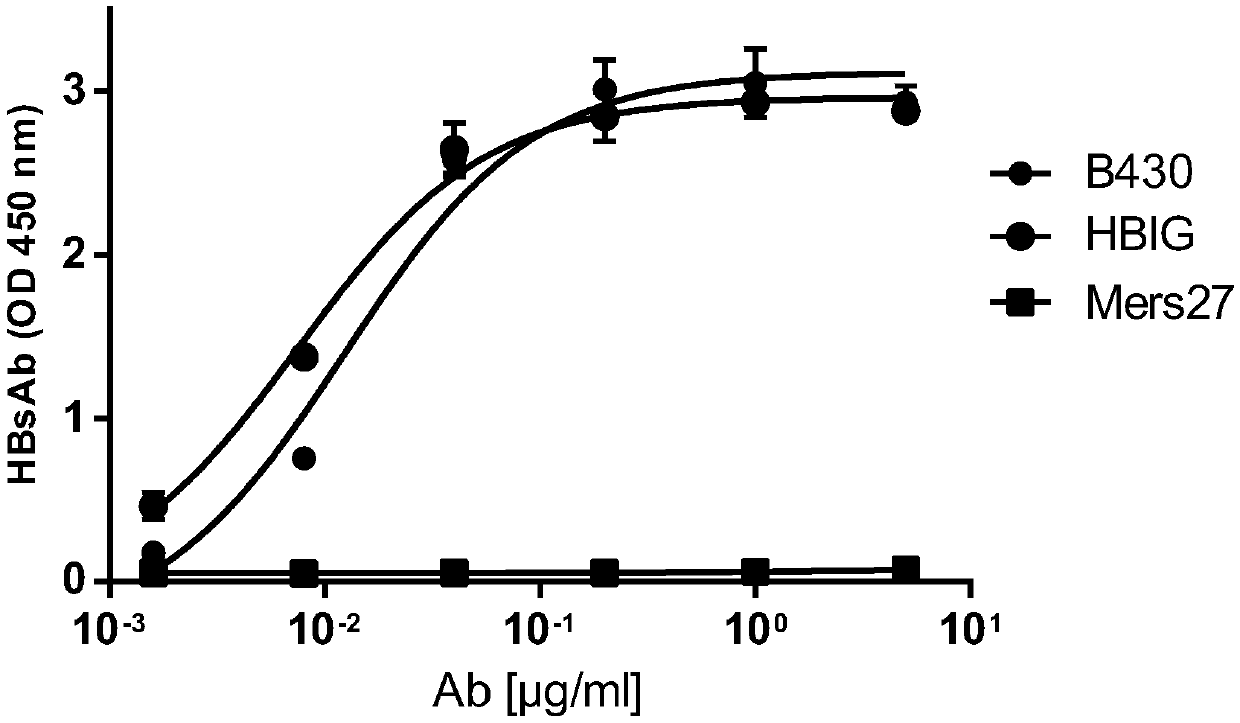
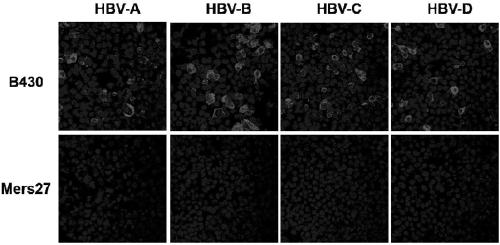
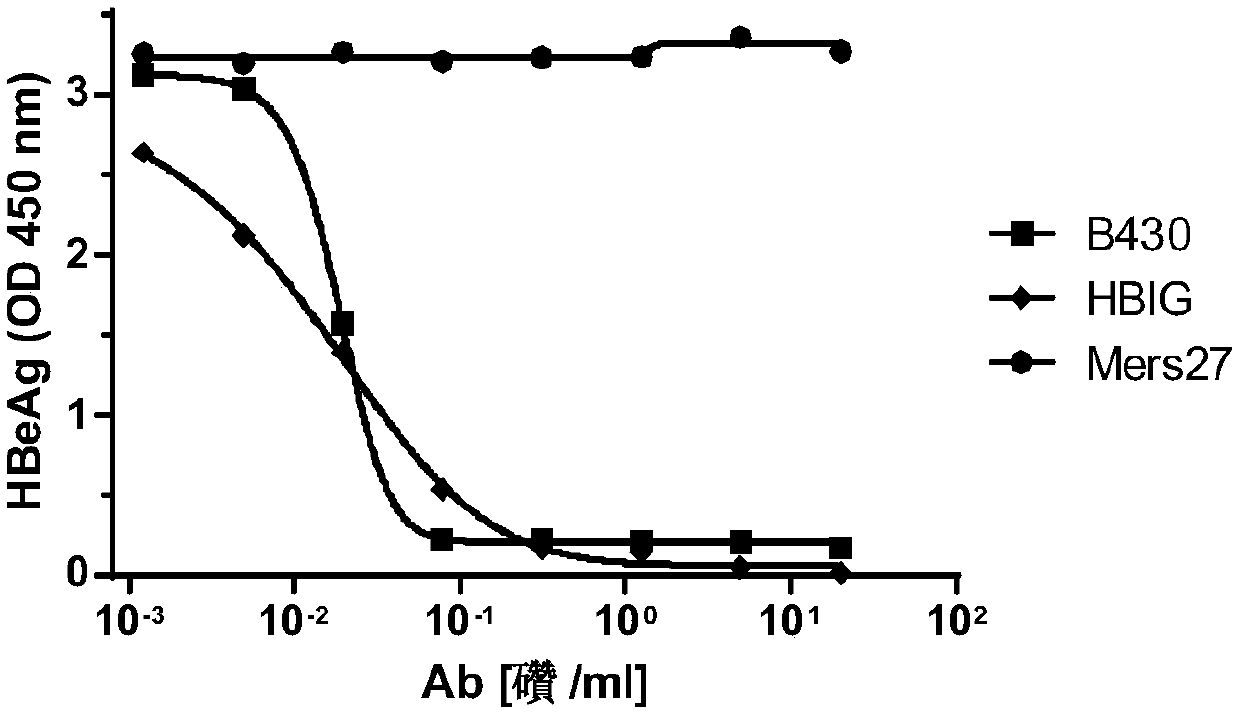
[0054] Embodiment 3, the binding ability of antibody and hepatitis B virus
[0055] 1. ELISA detection
[0056] 1. Take the ELISA plate and add the original coating solution for coating (50ng original coating / well).
[0057] Coating was originally HBs antigen. A coating stock solution was prepared using PBS buffer as a solvent.
[0058] 2. After completing step 1, take the ELISA plate, add 200 μl of PBS buffer solution containing 2% BSA to each well, incubate at 37°C for 2 hours to block, then discard the supernatant and wash twice with PBST solution.
[0059] 3. After completing step 2, take the ELISA plate, add 200 μl of antibody diluent to each well, incubate at 37°C for 1 hour, then discard the supernatant and wash twice with PBST solution. The antibody diluent is obtained by diluting the B430 antibody solution or the HBIG solution or the MERS-27 antibody solution prepared in Example 2 with PBS buffer. Five replicate wells were set up for each antibody dilution.
[00...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap