Method for checking total number of aerobic bacteria of ranitidine hydrochloride capsules
A technology of ranitidine hydrochloride and aerobic bacteria, which is applied in the field of medical inspection, can solve problems such as the interference of the total number of aerobic bacteria, and achieve stable and reliable detection results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
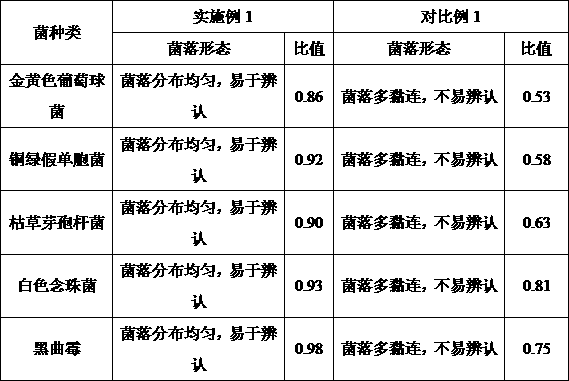
[0014] Take 10 g of ranitidine hydrochloride capsules and place them in a sterile Erlenmeyer flask, add pH 7.0 sterile sodium chloride-peptone buffer solution to dilute to 100ml, shake to disperse evenly, and prepare a 1:10 test solution. The 1:10 test solution in the shaped bottle is placed in the large-volume side of the special bag of the homogenizer with filter function, fully patted in the beating instrument for 30 seconds, and the test solution on the small-volume side after filtration is taken as the rough filter 1:10 For the test solution, take 20ml of the rough filter 1:10 test solution and dilute it to 100ml with pH7.0 sterile sodium chloride peptone buffer to make a 1:50 test solution, take 1ml of the 1:50 test solution, and add it to the plate Immediately inject 20ml of tryptone soy agar medium, mix well, solidify, culture at 33°C for 3 days, and measure the number of colonies.
experiment example 1
[0017] Experimental example 1 Applicability test of counting method
[0018] The comparison of the advantages and disadvantages of different inspection methods can be carried out according to the applicability test of the counting method in General Rule 1105 "Microbial Limit Test of Non-sterile Products: Microbial Enumeration Method" in the 2015 edition of the "Chinese Pharmacopoeia". Proceed as follows:
[0019] (1) Bacterial solution preparation
[0020] ① Staphylococcus aureus, Pseudomonas aeruginosa, Bacillus subtilis.
[0021] Take fresh cultures of Staphylococcus aureus, Pseudomonas aeruginosa, and Bacillus subtilis that have been cultured at 30-35°C for 18-24 hours, and use 0.9% sterile sodium chloride solution to make 5,000-10,000 cfu per 1 mL bacterial suspension;
[0022] ②Candida albicans
[0023] Take the fresh culture of Candida albicans cultured at 20-25°C for 2-3 days, and use 0.9% sterile sodium chloride solution to make a bacterial suspension containing ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com