Novel crystal form of sartan drug as well as preparation method and application thereof
A technology of sartans and drugs, which is applied in the direction of drug combination, organic chemical methods, and active ingredients of heterocyclic compounds, etc., can solve the problems of unfavorable oral solid preparation production and storage, restriction of drug application, poor water solubility, etc., and achieve physical and chemical drug properties Superior, environment-friendly, good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Preparation of co-crystal of sacubitril-valsartan trisodium trihydrate
[0043] Sacubitril-valsartan trisodium 2.5 hydrate co-crystal was prepared by referring to the method reported by Novartis in Chinese patent ZL200680001733.0 (PCT patent WO2007 / 056546)
[0044] Take 0.98401 g (1.027 mmol) of sacubitril valsartan trisodium 2.5 hydrate eutectic, spread it into a thickness of about 1 mm, place it at 25 ° C, 60 ± 2% relative humidity for 24 hours, collect the material, and obtain sacubitril Trivalsartan trisodium trihydrate co-crystal with quantitative molar yield.
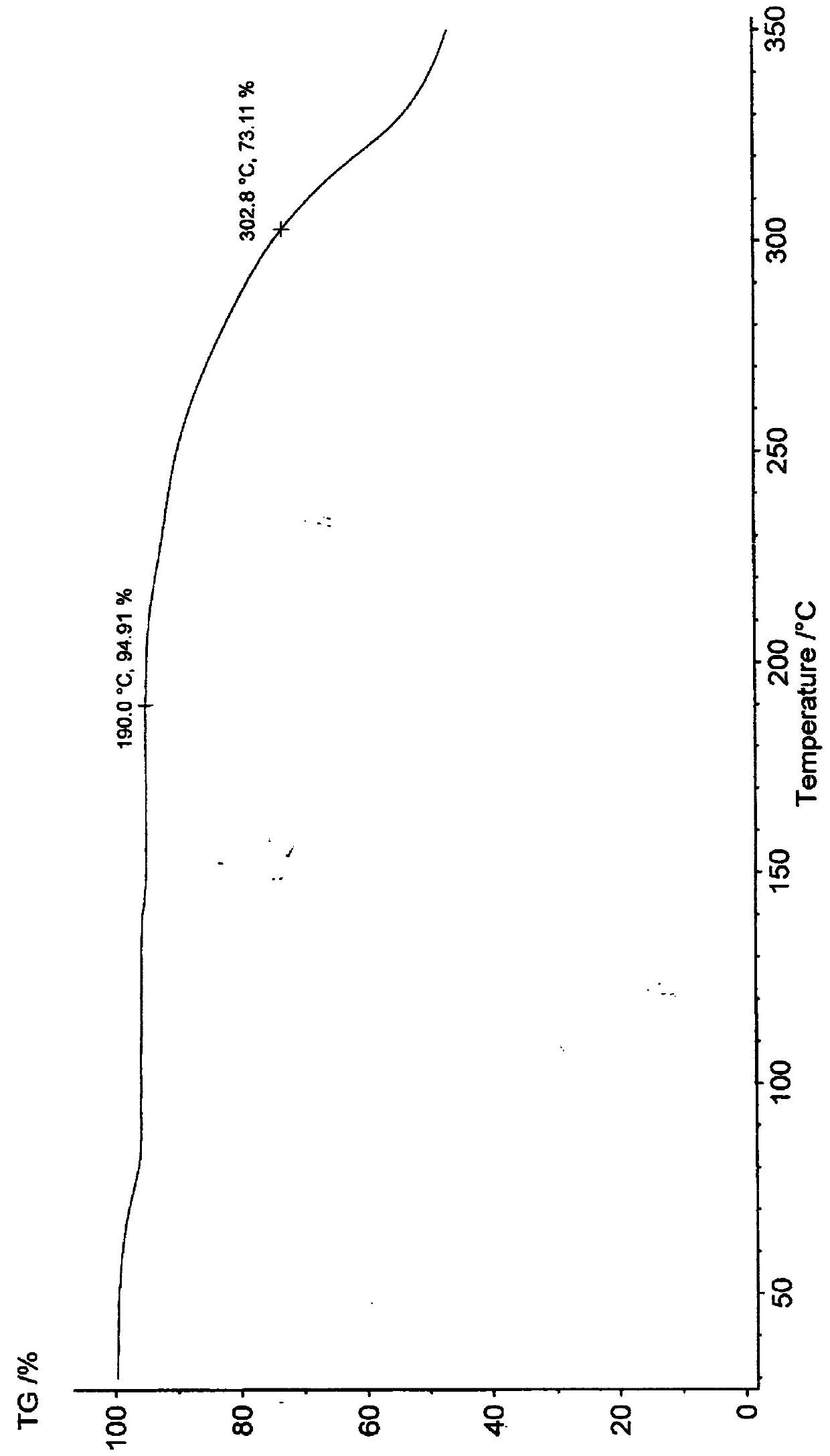
[0045] Karl Fischer method test moisture measured value (calculated value): 5.69% (5.58%).
[0046] Elemental analysis (C 48 h 61 N 6 Na 3 o 11 ) found (calculated value, %): C 59.83 (59.62), H 6.37 (6.36), N 8.73 (8.69).
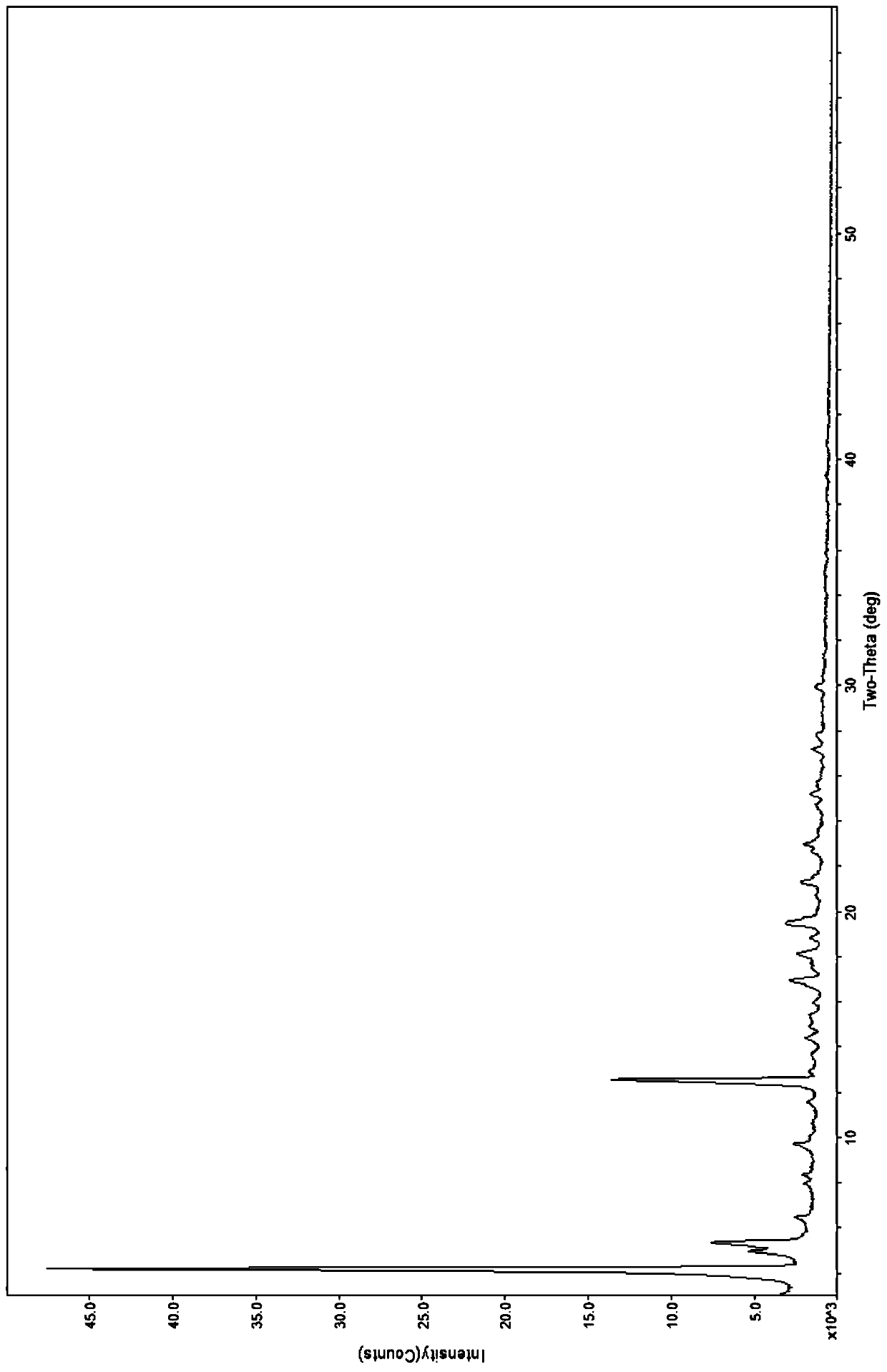
[0047] The obtained crystals were subjected to X-ray powder diffraction, using Cu target Kɑ rays, voltage 40kV, current 40mA, divergence slit 1 / 8°, anti-scatter slit 1 / 4°, anti-sc...
Embodiment 2
[0052] Preparation of co-crystal of sacubitril-valsartan trisodium trihydrate
[0053] Sacubitril-valsartan trisodium 2.5 hydrate co-crystal was prepared by referring to the method reported by Novartis in Chinese patent ZL200680001733.0 (PCT patent WO2007 / 056546)
[0054] Take 1.02736 g (1.072 mmol) of sacubitril valsartan trisodium 2.5 hydrate eutectic, spread it to a thickness of about 1 mm, place it at 25 ° C, 40 ± 2% relative humidity for 24 hours, collect the material, and obtain sacubitril Trivalsartan trisodium trihydrate co-crystal with quantitative molar yield.
[0055] Karl Fischer method test moisture measured value (calculated value): 5.67% (5.58%).
[0056]The obtained crystals were analyzed by X-ray powder diffraction spectrum, and the 2θ values of the characteristic absorption peaks were located at 4.2±0.2゜, 5.0±0.2゜, 5.4±0.2゜, 9.7±0.2゜, 12.6±0.2゜, 17.0±0.2゜, 18.1± 0.2°, 19.4±0.2°, 21.3±0.2°, 23.0±0.2°.
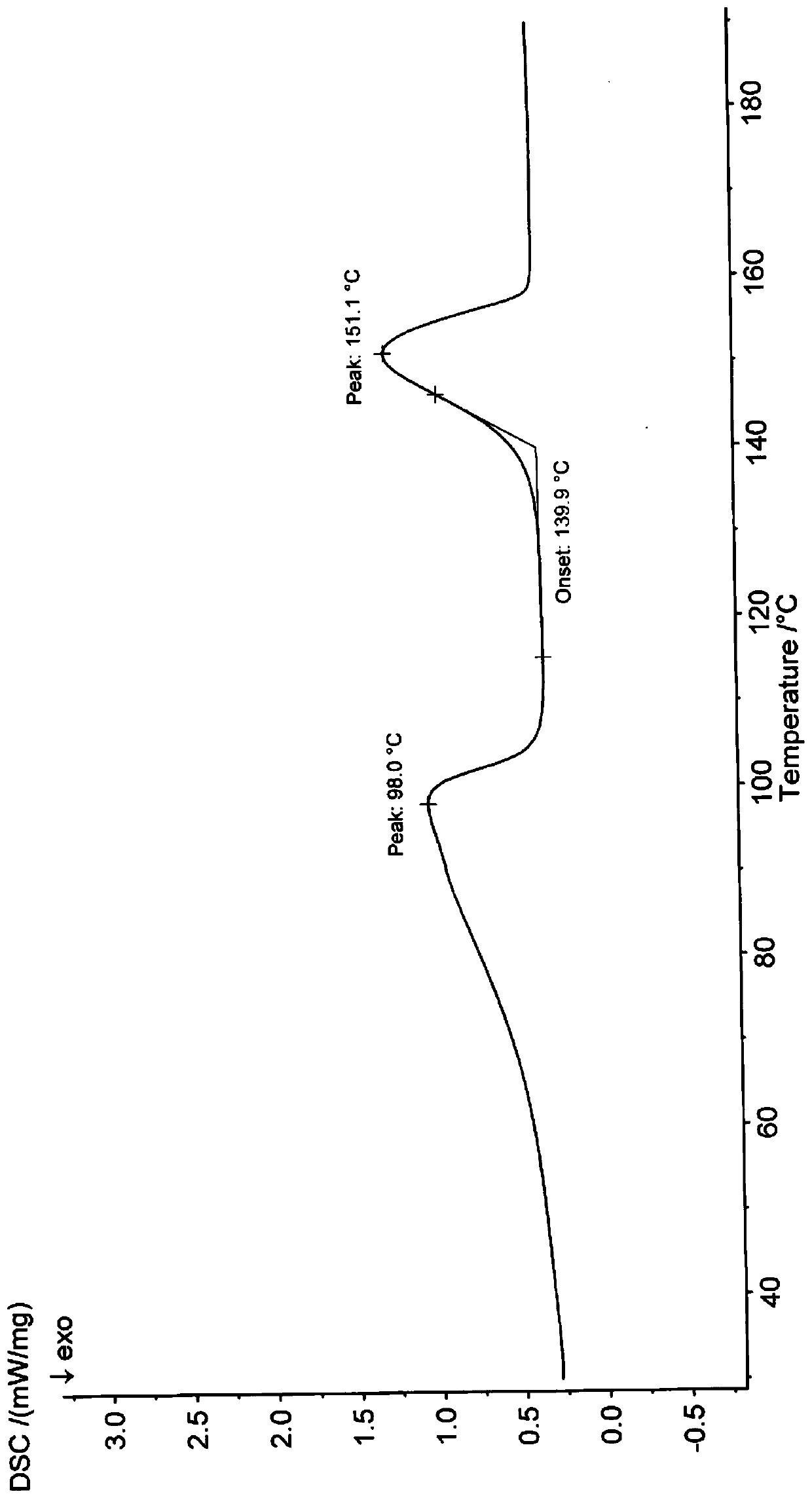
[0057] Differential scanning calorimetry (DSC) test ...
Embodiment 3
[0059] Preparation of co-crystal of sacubitril-valsartan trisodium trihydrate
[0060] Sacubitril-valsartan trisodium 2.5 hydrate co-crystal was prepared by referring to the method reported by Novartis in Chinese patent ZL200680001733.0 (PCT patent WO2007 / 056546)
[0061] Take 1.06124 g (1.108 mmol) of sacubitril valsartan trisodium 2.5 hydrate eutectic, spread it to a thickness of about 1 mm, place it at 35 ° C, 54 ± 2% relative humidity for 24 hours, collect the material, and obtain sacubitril Trivalsartan trisodium trihydrate co-crystal with quantitative molar yield.
[0062] Karl Fischer method test moisture measured value (calculated value): 5.55% (5.58%).
[0063] The obtained crystals were analyzed by X-ray powder diffraction spectrum, and the 2θ values of the characteristic absorption peaks were located at 4.2±0.2゜, 5.0±0.2゜, 5.4±0.2゜, 9.7±0.2゜, 12.6±0.2゜, 17.0±0.2゜, 18.1± 0.2°, 19.4±0.2°, 21.3±0.2°, 23.0±0.2°.
[0064] Differential scanning calorimetry (DSC) test...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com