A kind of gel, its complete set of raw materials and application
A gel, complete set of technology, applied in the direction of prosthesis, medical science, etc., can solve the problems of slow gel formation, unable to maintain a stable state, excessive swelling, etc., to reduce pain, reduce costs, and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
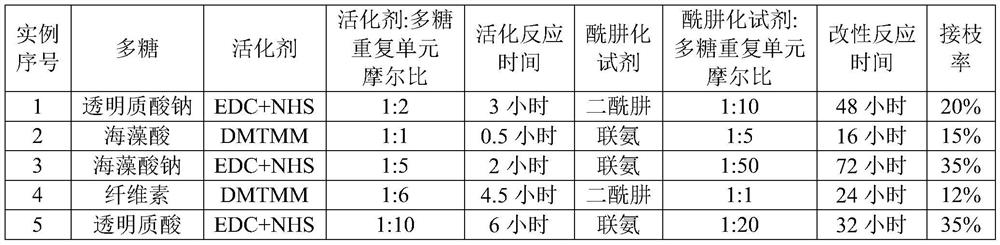
[0049] The raw material of the gel set in this example is composed of the first component and the second component, the first component is aldehyde-modified polysaccharide, and the second component is composed of hydrazide-based small molecule and hydrazide-modified polysaccharide , the mass ratio of hydrazide-based small molecule to hydrazide-modified polysaccharide is 1:100-100:1; the ring-opening rate of aldehyde-modified polysaccharide is 10-70%, and the grafting rate of hydrazide-modified polysaccharide 10-80%. Note: During specific preparation, the ring-opening rate / grafting rate can be controlled by controlling the feed ratio and reaction time.
[0050] The first component is prepared by the following method:
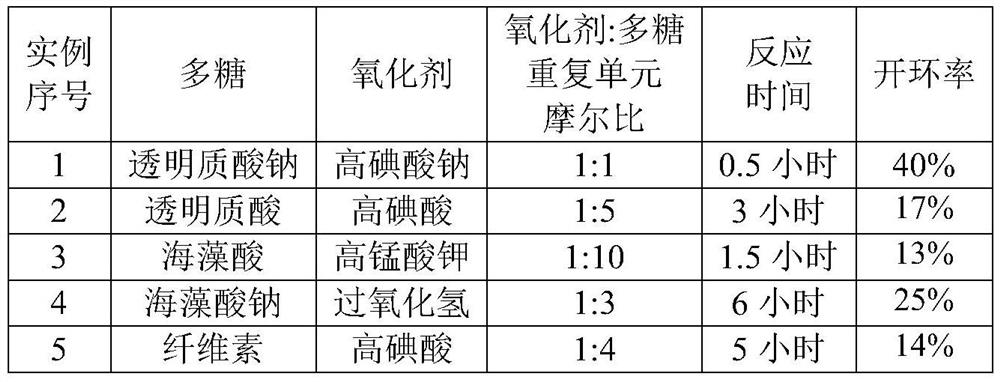
[0051] The polysaccharide used in the first component is dissolved in purified water to form a polysaccharide solution; the oxidizing agent is added to the polysaccharide solution and reacted in the dark; after the reaction is completed, the product is purified ...
Embodiment 2
[0066] The gel of this embodiment is obtained by preparing the raw materials of the gel set in Example 1 through the following steps:
[0067] S1. Dissolving the first component in physiological saline or buffer to form a first solution, and dissolving the second component in physiological saline or buffer to form a second solution;
[0068] S2. Blending the first solution and the second solution; in the mixed solution of the first solution and the second solution, the molar ratio of the aldehyde group to the hydrazide group is 1:10 to 10:1;
[0069] S3. The mixed solution solidifies into a gel as time goes by, and the finished gel is obtained.
[0070] Specifically, the mass concentration of the first component in the first solution is 0.1-20%; the mass concentration of the second component in the second solution is 0.1-10%.
[0071] The buffer is selected from acetate buffer, phosphate buffer, and borate buffer; the pH range of the buffer is 2 to 10; the first solution and ...
Embodiment 3
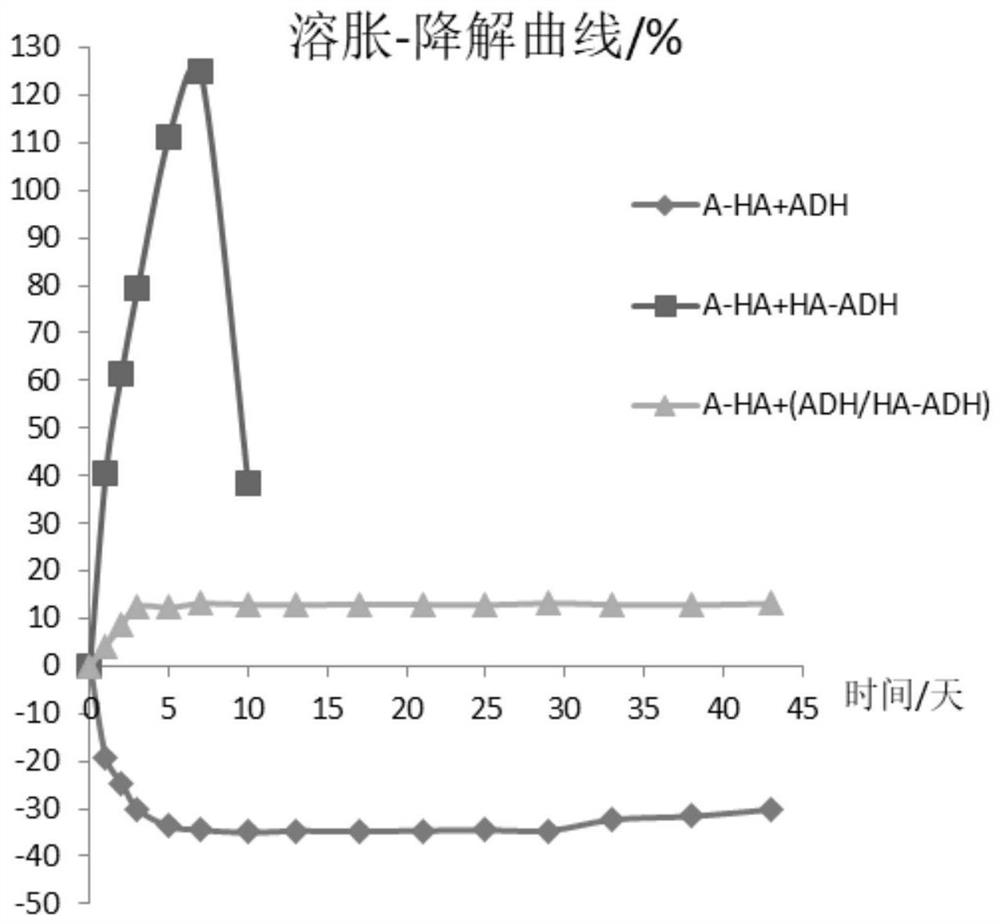
[0076] In this embodiment, a swelling test is performed on the gel to be tested.
[0077] The test samples are: Example 1 gel of Example 2, comparative gel 1, and comparative gel 2.
[0078] Compared with the example 1 gel of Example 2, the second component of the comparison gel 1 is only the hydrazide group small molecule of the example 1 gel, and all the other parameters are the same; the second component of the comparison gel 2 is only The hydrazide-modified polysaccharide of Example 1 gel, all the other parameters are the same.
[0079] The detection method is: after the hydrogel is formed, the initial hydrogel weight is measured as W 0 , then put the gel into 0.9% NaCl, under the condition of 37 ℃, take out the gel at different time points, blot the surface moisture, measure its weight as W i , according to the following formula to calculate its swelling rate.
[0080] Swelling rate = (W i -W 0 ) / W 0 ×100%
[0081] The result is as figure 1 shown. This result sho...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of grafting | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com