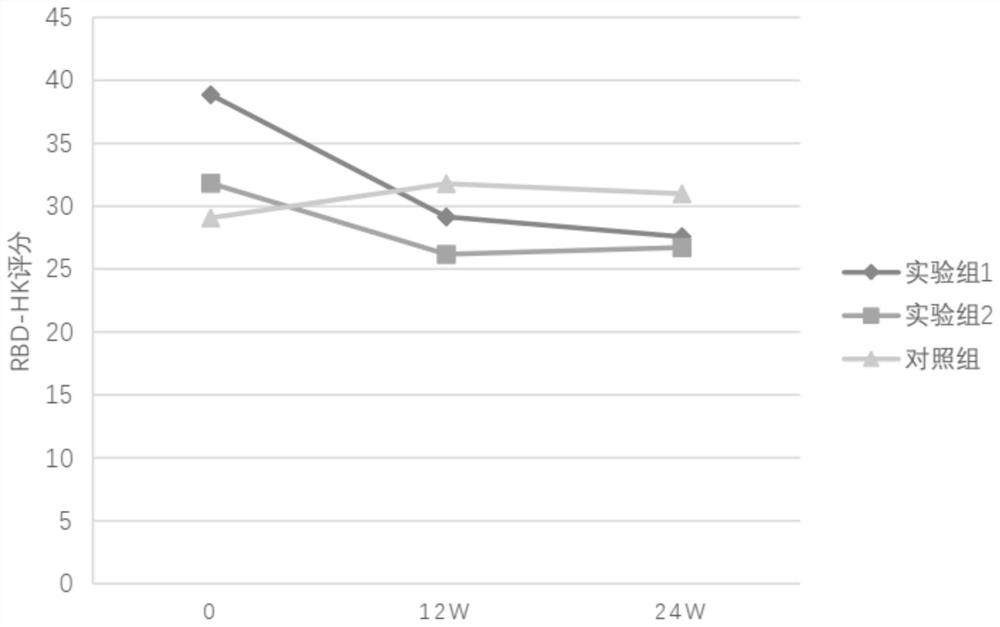
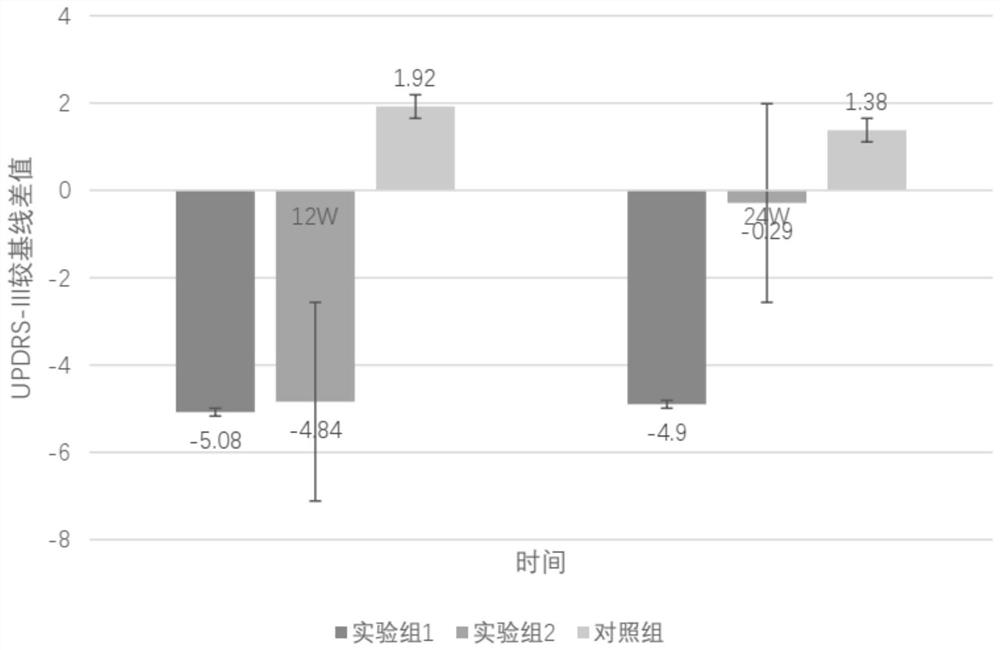
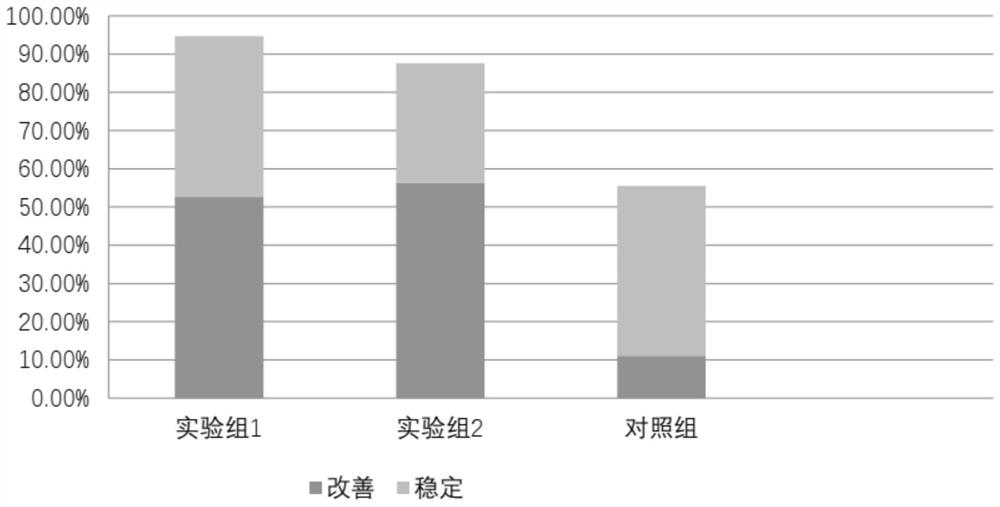
Probiotic composition, preparation and use for treating Parkinson's disease REM sleep behavior disorder
A probiotic preparation, a technology for Parkinson's disease, which can be used in drug combinations, medical preparations containing active ingredients, bacteria, etc., can solve the problems of increasing family care pressure, even hallucinations, and aggravating RBD, and achieve good clinical application prospects and Market prospect, reduced dosage, good protective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0054] The preparation method of the frozen powder is a conventional technique, and it can be referred to known techniques such as CN110367543A, CN110720638A, CN102356912B.
[0055] The probiotic composition, preparation method and colony standard of the present invention are in line with the "China Pharmacopoeia" 2010 Edition and China Biological Product Procedure 2000 Edition.
[0056] The CFU / G of the present invention is the number of colonies contained in each G detection sample, and the CFU is a colony formation unit. The CFU / G described in the following examples generally refers to "equal to" or "not less than", in the actual use in the actual use of the number of live bacteria in the shelf life, in the effective range and the patients in the intestinal medium, the general product The number of bacteria is "not less than".
[0057] The following examples of the present invention have prepared for probiotic capsules, and the equipment used, including 3D moving mixers (Ji...
Embodiment 1
[0058] Example 1 Preparation of a probiotic preparation of the present invention
[0059] (1) Dry lyophilized germacte and excipient powder and excipient powder and excipients of Bacillus, Long Bacillus, Eosinophilus, and Swyncus are mixed; in this example, the excipient starch And lactose;
[0060] (2) Package (1) to the obtained mixture, the capsule is made into a capsule, and the capsule of the capsule is an enteric capsule, and each capsule contains 250 mg of mixed bacteria.
[0061] The live bacteria containing each capsule is not less than: Acid Bacillus 10 × 10 8 CFU / G, Long Bifurcum 4.0 × 10 8 CFU / G, Eosin Treatment 1.0 × 10 8 CFU / G, Swift Fungus 0.5 × 10 8 CFU / g.
[0062] After satisfying the number of live bacteria, the excipient is a margin, the mass ratio of the pregerative starch and lactose is 1: 1.
Embodiment 2
[0063] Example 2 Preparation of a probiotic preparation of the present invention
[0064] (1) Dry lyophilized germacte and excipient powder and excipient powder and excipients of Bacillus, Long Bacillus, Eosinophilus, and Swyncus are mixed; in this example, the excipient starch And lactose;
[0065] (2) Package (1) to the obtained mixture, the capsule is made into a capsule, and the capsule of the capsule is an enteric capsule, and each capsule contains 250 mg of mixed bacteria.
[0066] The live bacteria containing each capsule is not less than: Acid Bacillus 10 × 10 9 CFU / G, Long Bifurcum 4.0 × 10 9 CFU / G, Eosin Treatment 1.0 × 10 9 CFU / G, Swift Fungus 0.5 × 10 9 CFU / g.
[0067] After satisfying the number of live bacteria, the excipient is a margin, the mass ratio of the pregerative starch and lactose is 1: 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com