Method for synthesizing leaf alcohol from m-pentadiene
A technology for piperylene and leaf alcohol is applied in the field of synthesizing leaf alcohol from piperylene, which can solve the problem that the conversion rate of piperylene is low, the yield of intermediate selectivity leaf alcohol is low, the recovery of alkali metals and amines is not easy, and the recovery of leaf alcohol is difficult. The equipment is corrosive and other problems, so as to achieve the effect of convenient and easy-to-obtain raw materials, cheap raw materials, and low equipment requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] The preparation of catalyst 1a is carried out according to the following sequence of steps:
[0053] At 30°C, in a stainless steel autoclave, add 30 g of 1-(2-piperidinyl) ethyl-3-methylimidazolium hexafluorophosphate and 1 g of RuCl 3 .3H 2 O, the nitrogen pressure is 1.0 MPa, the stirring speed is 800 rpm, and the reaction is carried out for 4 hours. After the end, the pressure was released and cooled to room temperature to obtain a catalyst, which was designated as catalyst 1a.
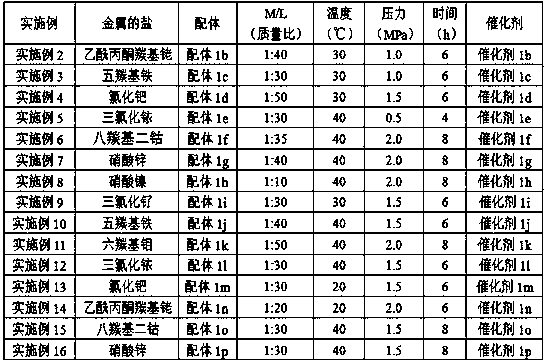
Embodiment 2-16
[0055] The operation steps are consistent with Example 1, and the specific data are shown in Table 1. Nitrogen-containing functionalized ionic liquids selected from 1-(2-piperidinyl)ethyl-3-methylimidazolium hexafluorophosphate (ligand 1a), 1-(2-piperidinyl)ethyl-2 , 3-dimethylimidazolium hexafluorophosphate (ligand 1b), 1-(2-piperidinyl)ethyl-3-methylimidazolium tetrafluorophosphate (ligand 1c), 1-(2-piperidinyl Pyridyl)ethyl-2,3-dimethylimidazolium tetrafluorophosphate (ligand 1d), 1-(2-piperidinyl)ethyl-3-methylimidazolium acetate (ligand 1e), 1-(2-piperidinyl)ethyl-2,3-dimethylimidazole acetate (ligand 1f), 1-(2-piperidinyl)ethyl-3-methylimidazole methanesulfonate (ligand 1g), 1-(2-piperidinyl)ethyl-2,3-dimethylimidazolium methanesulfonate (ligand 1h), 1-(N-morpholinyl)ethyl-N- Methylpiperazine hexafluorophosphate (ligand 1i), 1-(N-morpholinyl)ethyl-N,N-dimethylpiperazine hexafluorophosphate (ligand 1j), 1-(N- Morpholinyl) ethyl-N-methylpiperazine tetrafluorophosphate (...
Embodiment 17
[0061] 1. Synthesis of Intermediates
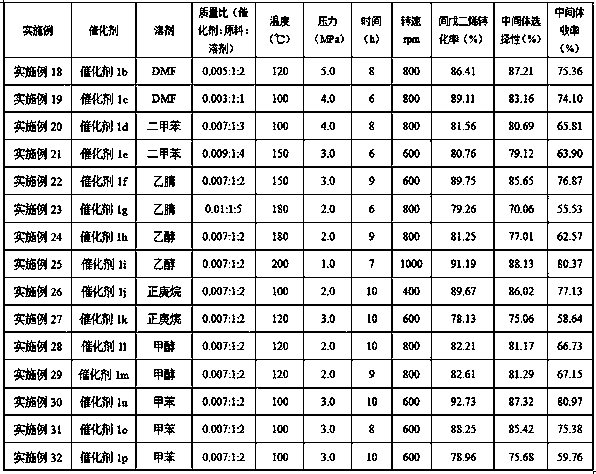
[0062] Add piperylene, catalyst 1a (the mass ratio of catalyst 1a to piperylene is 0.07:1) and solvent DMF (the mass ratio of piperylene to DMF is 1:3) into the autoclave, start stirring, and stir The speed is 800rpm, the syngas (CO:H 2 =1:1.2) The pressure is 2.0MPa, the temperature is raised to 100°C, and the reaction is kept for 8 hours. Sampling was detected by GC, the residual amount of piperylene was <2.0%, cooled to room temperature, the reaction product was derived, separated, and the reaction product cis-3-hexen-1-al was obtained.
[0063] The CO:H 2 =1:1.2, for CO and H 2 The volume ratio is 1:1.2;
[0064] The conversion rate of piperylene was 90.56%, the selectivity of cis-3-hexen-1-al was 86.45%, and the yield was 78.29%.
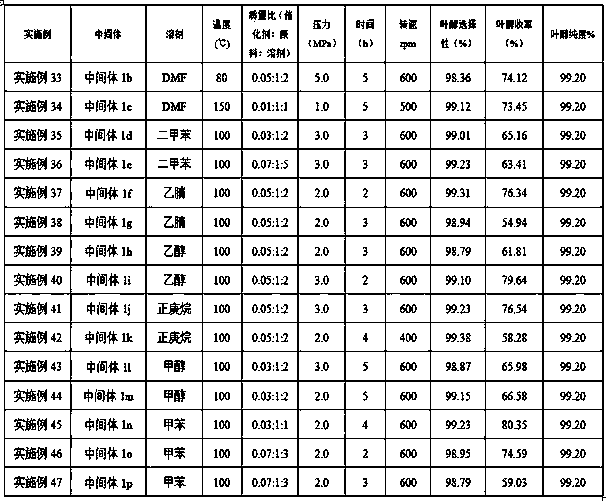
[0065] 2. Leaf alcohol synthesis
[0066] The cis-3-hexene-1-aldehyde intermediate, catalyst 3%Ru-1%Fe / Al 2 o 3 (catalyst 3%Ru-1%Fe / Al 2 o 3 The mass ratio of intermediate and intermediate is 0.0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com