Method for recycling acid in synthesis of 2-nitro-4-methylsulfonylbenzoic acid
A technology for thiamphenicol benzoic acid and acid recovery, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve unfavorable industrialized production, safe and stable progress, restriction of enterprise and social and economic development, existence safety and environmental protection Risk and other issues, to achieve the effect of solving safety and environmental risks, solving difficult to handle, and reducing the unit consumption of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
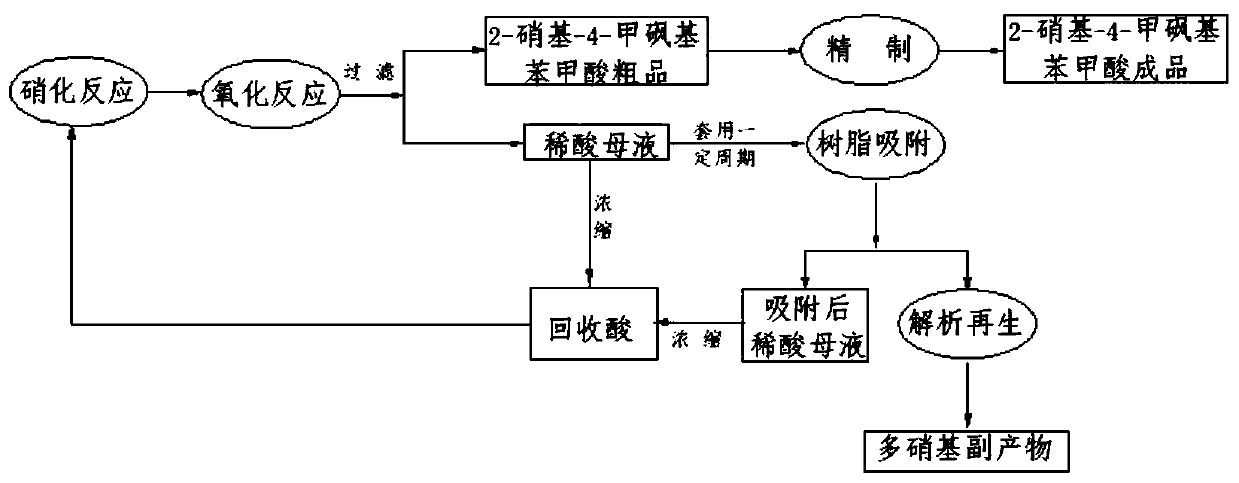
[0031] After filtering the crude 2-nitro-4-thiamphenicol benzoic acid, the dilute acid mother liquor is concentrated to 170-180°C under the condition of vacuum degree of 0.095-0.1MPa to obtain recovered acid with a concentration of 90%-98%, which is continuously circulated until After the 4th cycle, get filtered dilute acid mother liquor 600g, wherein the content of polynitro-substituent is 6012ppm after analysis. Use a measuring cylinder to measure 40ml of SP700 adsorption resin, and transfer it into two adsorption columns respectively, keep it firm, and keep a 3-5cm water layer on the upper layer of the resin. Connect the diluted acid mother liquor to be adsorbed, the peristaltic pump, and the two adsorption columns with rubber tubes. At 5-10°C, the peristaltic pump controls the flow rate to 0.5-1BV / h, so that the diluted acid mother liquor passes through the adsorption column, and then washes the adsorption column with 80ml of water, and the effluent is combined with the di...
Embodiment 2
[0036] After filtering the crude 2-nitro-4-thiamphenicol benzoic acid, the dilute acid mother liquor is concentrated to 170-180°C under the condition of vacuum degree of 0.095-0.1MPa to obtain recovered acid with a concentration of 90%-98%, which is continuously circulated until After the 6th cycle, get filtered diluted acid mother liquor 600g, wherein the content of nitro substitute is 7203ppm after analysis. Use a measuring cylinder to measure 40ml of SP825L adsorption resin, and transfer it into two adsorption columns respectively, keep it firm, and keep a 3-5cm water layer on the upper layer of the resin. Connect the dilute acid to be adsorbed, the peristaltic pump, and the two adsorption columns with rubber tubes. At 20-30°C, the peristaltic pump controls the flow rate to 2-2.5BV / h, so that the dilute acid mother liquor passes through the adsorption column, and then washes the adsorption column with 120ml of water, and the effluent is combined with the diluted acid mother...
Embodiment 3
[0041] After filtering the crude 2-nitro-4-thiamphenicol benzoic acid, the dilute acid mother liquor is concentrated to 170-180°C under the condition of vacuum degree of 0.095-0.1MPa to obtain recovered acid with a concentration of 90%-98%, which is continuously circulated until After the 5th cycle, get filtered diluted acid mother liquor 600g, wherein the content of nitro substituent is 6872ppm after analysis. Use a graduated cylinder to measure 40ml of SP207 adsorption resin, and transfer it into two adsorption columns respectively, keep it firm, and keep a 3-5cm water layer on the upper layer of the resin. Connect the dilute acid to be adsorbed, the peristaltic pump, and the two adsorption columns with rubber tubes. At 55-60°C, the peristaltic pump controls the flow rate to 3-4BV / h, so that the diluted acid mother liquor passes through the adsorption column, and then washes the adsorption column with 100ml of water, and the effluent is combined with the diluted acid mother ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com