Humanized cytokine animal model, preparation method and application
A humanized, non-human animal technology, applied in the field of biomedicine, can solve problems such as defects in the development and function of human cells, inability to screen and evaluate drug efficacy and defects in ordinary mice
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0199] Example 1 IL-6 Gene Humanized Mice
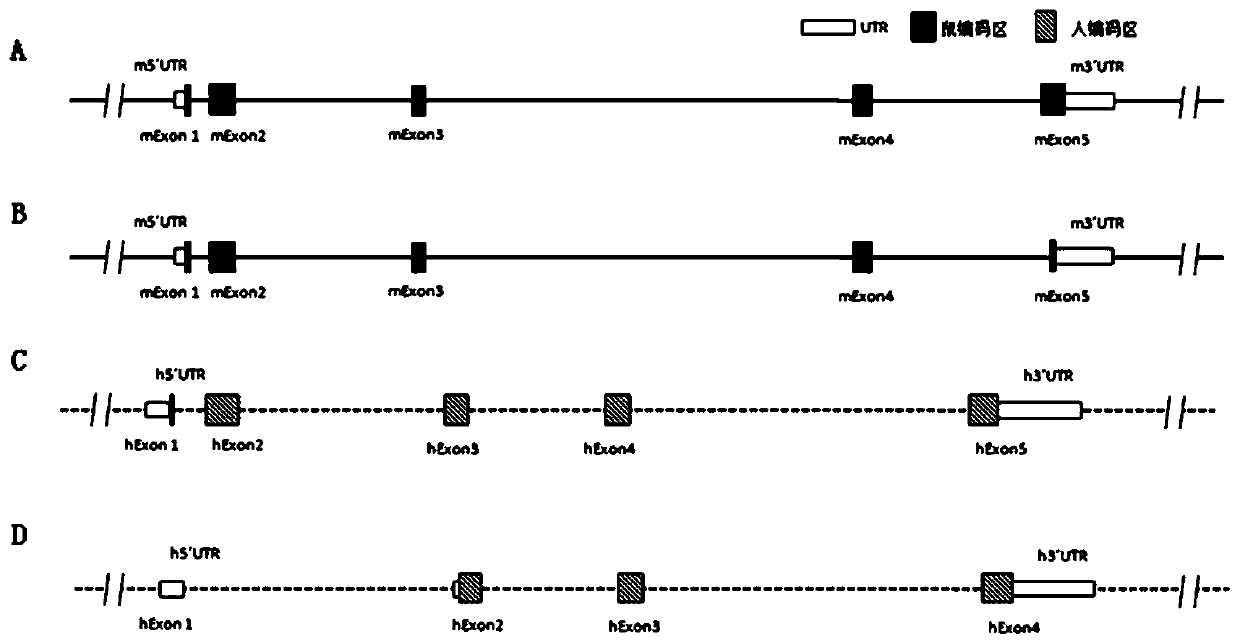
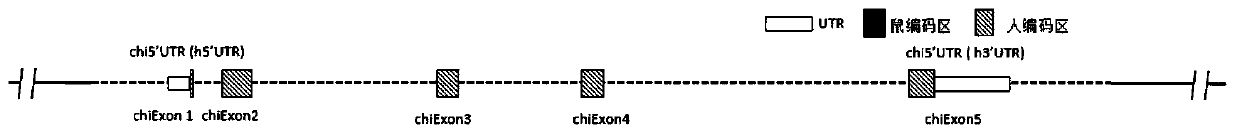
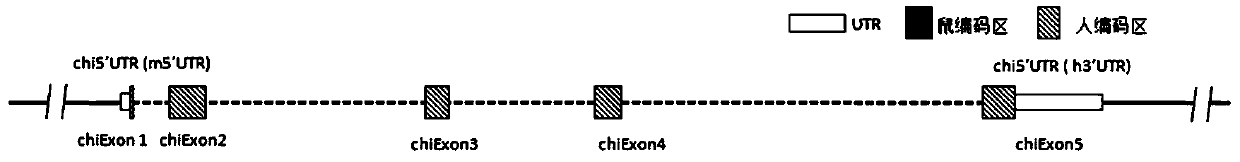
[0200] Mouse IL-6 gene (NCBI Gene ID: 16193, Primary source: MGI: 96559, UniProt ID: P08505, located at positions 30013114 to 30019975 of chromosome 5 NC_000071.6) and human IL-6 gene (NCBI Gene ID: 3569 , Primary source: HGNC: 6018, UniProt ID: P05231, located at positions 22725889 to 22732002 of chromosome 7 NC_000007.14), given that human IL-6 and mouse IL-6 have multiple subtypes or transcripts, such as figure 1 The gene schematic diagram shown demonstrates the inclusion of figure 1A: mouse transcript NM_031168.2 (SEQ ID NO: 1) and its encoded protein NP_112445.1 (SEQ ID NO: 2), figure 1 B: mouse transcript NM_001314054.1 (SEQ ID NO: 3) and its encoded protein NP_001300983.1 (SEQ ID NO: 4), figure 1 C: human transcript NM_000600.4 (SEQ ID NO: 5) and its encoded protein NP_000591.1 (SEQ ID NO: 6), figure 1 D: Human transcript NM_001318095.1 (SEQ ID NO: 7) and its encoded protein NP_001305024.1 (SEQ ID NO: 8).
[0201] In order...
Embodiment 2
[0263] Example 2 IL-6R Gene Humanized Mice
[0264] Mouse IL-6R gene (NCBI Gene ID: 16194, Primary source: MGI: 105304, UniProtID: P08505, located at positions 89869324 to 89913196 of chromosome 3 NC_000069.6) and human IL-6R gene (NCBI Gene ID: 3570, Primary source: HGNC: 6019, UniProt ID: P08887, located at positions 154405193 to 154469450 of chromosome 1 NC_000001.11), given that human IL-6R and mouse IL-6R have multiple subtypes or transcripts, such as Figure 13 The gene schematic diagram shown is based on mouse transcript NM_010559.3 (SEQ ID NO: 59) and its encoded protein NP_034689.2 (SEQ ID NO: 60), human transcript NM_000565.3 (SEQ ID NO: 61) and its encoded protein Take protein NP_000556.1 (SEQ ID NO: 62) as an example.
[0265] In order to achieve the purpose of the present invention, the gene sequence encoding human IL-6R protein can be introduced into the endogenous mouse IL-6R locus, so that the mouse expresses human IL-6R protein. For example, mouse embryonic ...
Embodiment 3
[0294] Example 3 Reconstitution of the human immune system in IL-6 humanized mice
[0295] Using the IL-6 humanized mouse (B-NDG background) prepared in Example 1 as a tool can transplant human CD34+ cells and realize the reconstruction of the human immune system. Umbilical cord blood stem cells injected into the tail vein of mice after myeloablation by irradiation. Plasma samples were collected and tested at various times after transplantation. The results showed that IL-6 humanized mice (B-NDG background) had higher human peripheral blood engraftment than wild-type controls, and the percentage of differentiation was closer, containing some mature B cells.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com