Bifunctional angiogenesis inhibitor and use thereof
An angiogenesis-inhibiting, dual-function technology, used in epidermal growth factors, cardiovascular system diseases, medical preparations containing active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1: Selection of host cells
[0047] Dihydrofolate reductase (DHFR) gene-deficient Chinese hamster ovary cells (Chinese Hamster OvaryCell, CHO / dhfr-) were purchased from ATCC, USA, catalog number CRL-9096, lot number (Lot number) 3691620. CHO / dhfr-cells were cultured in IMDM complete medium supplemented with hypoxanthin and thymidine (HT) and 10% fetal bovine serum (FBS). The cells were polygonal and adhered to the wall. Nitrogen freezer. In order to obtain CHO / dhfr-cells adapted to serum-free suspension culture, one of the frozen cells was taken, thawed in a water bath at 37°C, and the cells were suspended in Ex-Cell 302CHO medium (Sigma) containing 10% FBS and HT. Let the cells adhere to the wall and grow in the cell culture flask. After the cells grow well, suspend the cells in 30 mL of Ex-Cell 302 CHO medium containing 5% fetal bovine serum, and culture in shake flasks. When the cells grow to 1-2×10 6 / mL, transferred to Ex-Cell 302 CHO cell culture medium...
Embodiment 2
[0048] Example 2: Target gene
[0049] The RC28-05 fusion protein is a fusion protein with bifunctional angiogenesis inhibitory activity, which is composed of partial amino acid sequences of VEGFR and FGFR, fused with the Fc fragment of human immunoglobulin, and its amino acid sequence is as follows (such as SEQ ID NO: 1 shown):
[0050]
[0051] The nucleotide sequence of RC28-05 is as follows (1923bp) (as shown in SEQ ID NO:3):
[0052]
[0053]
[0054] After introducing double restriction sites at both ends of the RC28-05 target gene sequence and inserting a conventional expression vector, the general method was used to transfect CHO cells, and the RC28-05 expression cell line was screened out to express the RC28-05 fusion protein.
Embodiment 3
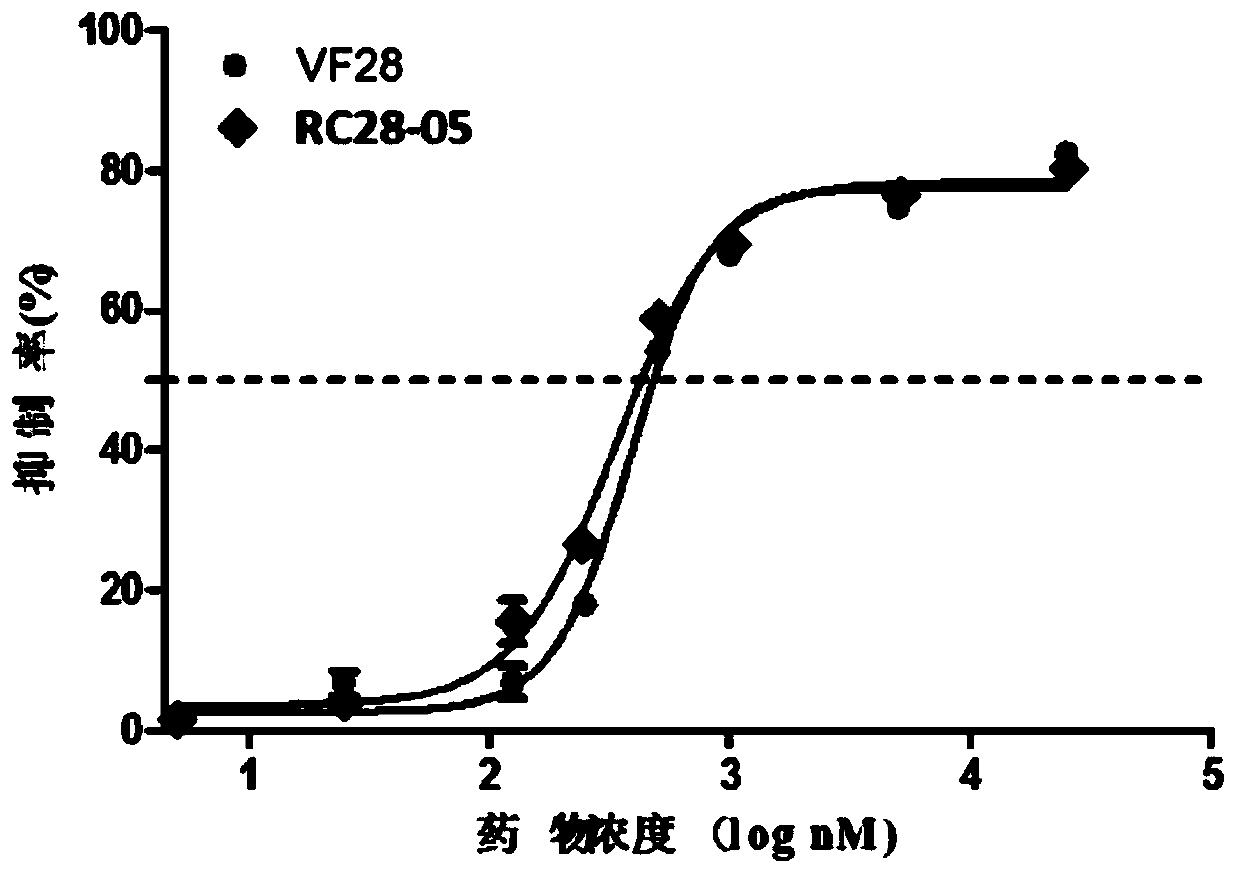
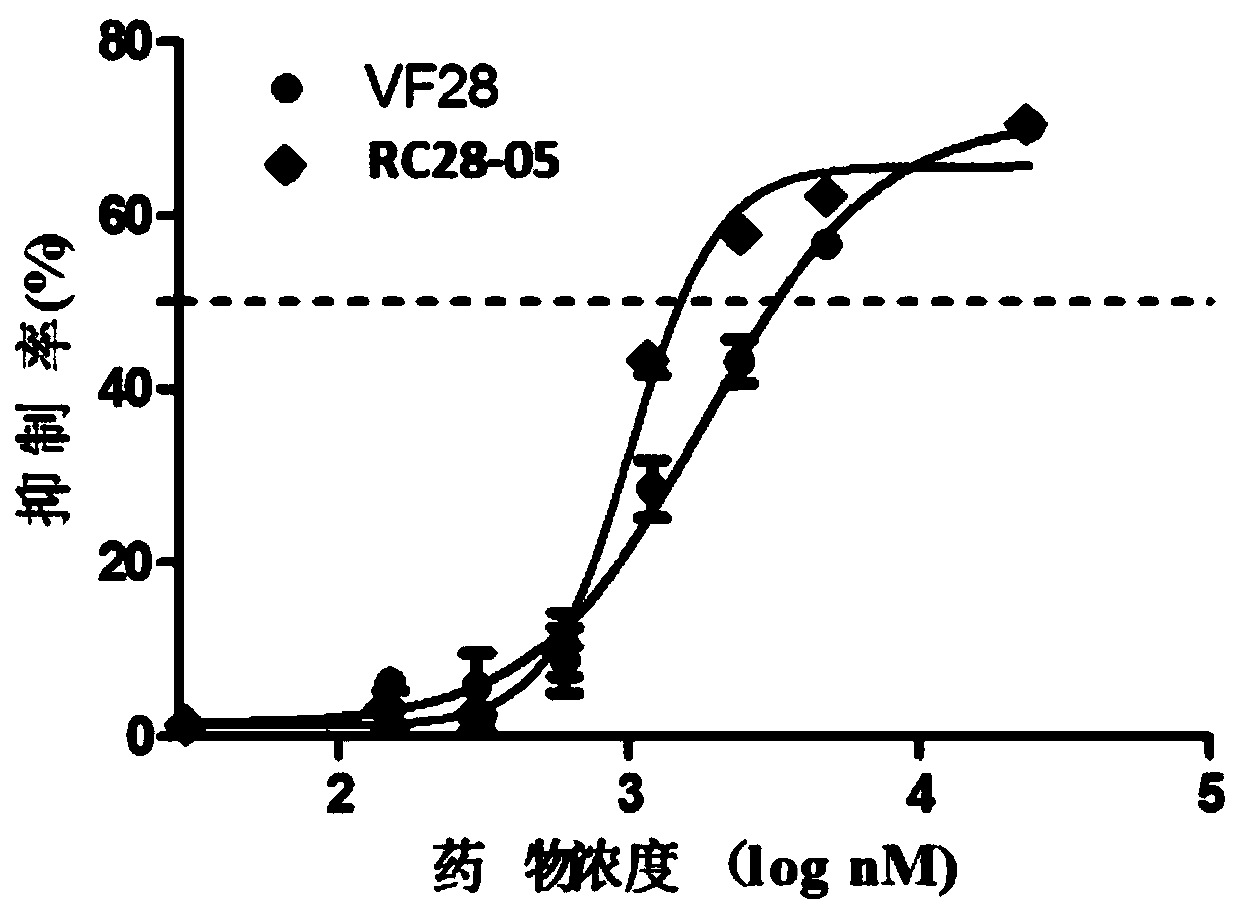
[0055] Embodiment 3: the affinity experiment of fusion protein
[0056] Use ForteBio Octet (PALL company) to detect the affinity of VF28 and RC28-05, add PBS (pH7.4) to each well of the first column of the test plate A-E row as the balance solution, soak the probe for 10min to activate; dilute RC28 with PBS respectively -05 and VF28 to a concentration of 50nM, add the 2nd column of the A-E row of the 96-well assay plate, set the program as Loading, 300s; add PBS to each well of the 3rd column of the test plate A-E row as the balance solution, set the program as Baseline, 180s ; Using PBS as the diluent, dilute rhVEGF (R&D) and rhFGF (R&D) to concentrations of 500nM and 400nM, respectively, and then serially dilute 3 gradients (total of four gradients) at 1:2 to concentrations of 62.5nM and 50nM , add the serially diluted samples to the 4th column of row A-D of the 96-well plate, and add the diluted solution to the 4th column of E row as a negative control, set the program to A...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com