B cell epitope polypeptide of serine protease inhibitor (Ts-WM5) in larvae phase of trichina muscle, hybridoma cell strain, monoclonal antibody and application thereof
A hybridoma cell line and monoclonal antibody technology, applied in the field of immunology, can solve problems such as hindering practical application, cumbersome preparation, and uneven batch quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
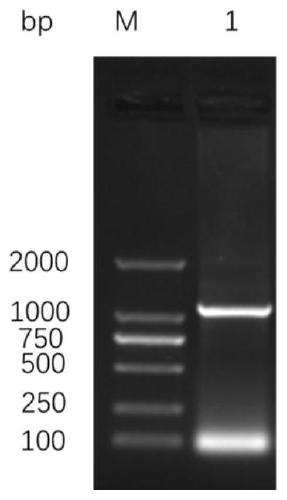
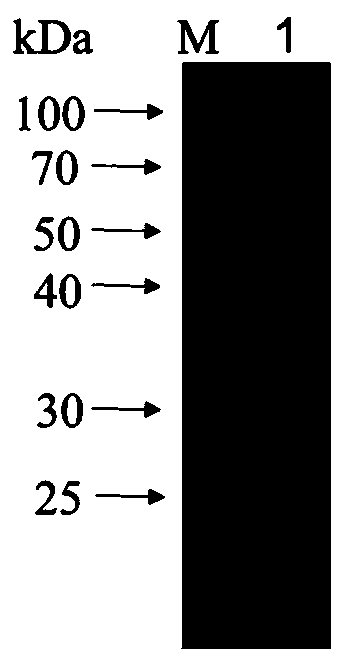
[0063] Example 1 Prokaryotic expression and purification of Ts-WM5 protein
[0064] 1. Primer Design
[0065] According to the registered WM5 gene sequence in Genbank (accession number: DQ864973.2), design PCR amplification primers, the sequence is as follows:
[0066] WM5-HindIII-atg:5'-ATAC AAGCTT ATGGAAACAGAAATTGCAAA-3' (as shown in SEQ ID No.3)
[0067] WM5-XhoI-tta:5'-ATTAA CTCGAG AATTACCAGAAAAACGTCCAA-3' (as shown in SEQ ID No.4)
[0068] The underlined part is the introduced HindIII and XhoI restriction sites, and the expected length of the amplified product is 1238bp.
[0069] 2. Extraction and reverse transcription of Trichinella spiralis T1 (T.spiralis) RNA
[0070] Take one Trichinella spiralis T1 (T.spiralis) conservation mouse in this experiment, kill it by neck dislocation, peel off the skin, tail, viscera and claws, wash the carcass and mince it, put it in 300ml containing 1% HCl and 1% stomach The protease digestion solution was stirred and digested in ...
Embodiment 2
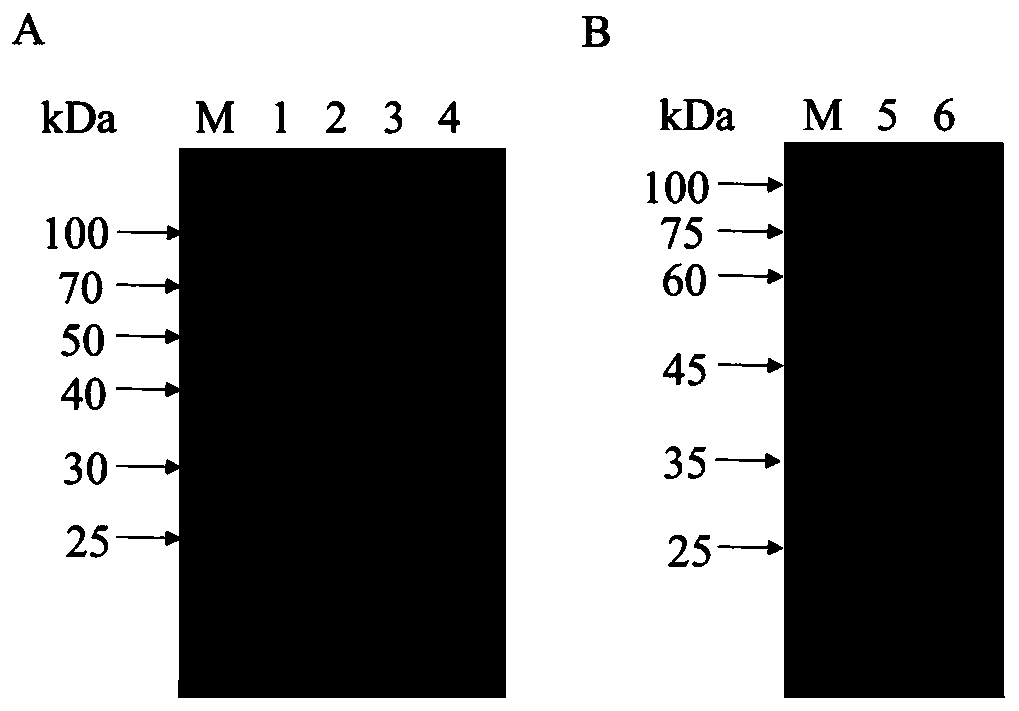
[0089] Example 2 Preparation of anti-Ts-WM5 protein monoclonal antibody
[0090] 1. Mice Immunization
[0091] Five 6-week-old female BALB / c mice were immunized with recombinant Ts-WM5 protein purified from gel cutting, and immunized five times with a two-week interval between each immunization. The immunization dose was 50 μg / mouse, and the immunization route was intraperitoneal immunization.
[0092] One week after the 4th immunization and the 5th immunization, blood was collected from the tail of the mice, and the serum was separated (4°C, centrifuged at 3000rpm for 30min), and the antibody level was detected by Ts-WM5 indirect ELISA method. The Ts-WM5 indirect ELISA method is operated as follows: the Ts-WM5 protein purified on the column is diluted with a coating solution (0.01M NaOH PH12), and the coating amount is 0.125ug / well, 100ul per well. Coat at 37°C for 2h or overnight at 4°C. After that, the plate was washed 3 times with PBST (containing 0.05% Tween 20). Block...
Embodiment 3
[0100] Identification of embodiment 3 monoclonal antibody
[0101] 1. Subclass identification of monoclonal antibodies
[0102] Subclass identification of the monoclonal antibody obtained in Example 2 was carried out according to the operating instructions of the antibody subclass identification kit. The results show that the heavy chain of the monoclonal antibody of the present invention is IgG1 and the light chain is κ chain, see Table 1 for details.
[0103] Table 1 Identification of monoclonal antibody subclasses
[0104]
[0105]
[0106] 2. Ts-WM5 competition ELISA test
[0107] Coating and blocking are the same as Ts-WM5 indirect ELISA, starting from 1:1, diluting the positive and negative sera of Trichinella spiralis infection by 2 times from 1:1, taking 50 μl of the diluted serum to be tested and mixing it with 50 μl of the monoclonal antibody supernatant respectively, and taking After mixing, 100 μl of the solution was added to a microwell plate and reacted ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com