Methods of administering intravenous meloxicam pre-operatively and in combination with other drugs
A meloxicam, intravenous technology, applied in the field of intravenous administration of meloxicam before surgery and in combination with other drugs, can solve the problems of serious complications, difficult side effects, prolonged hospital stay, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
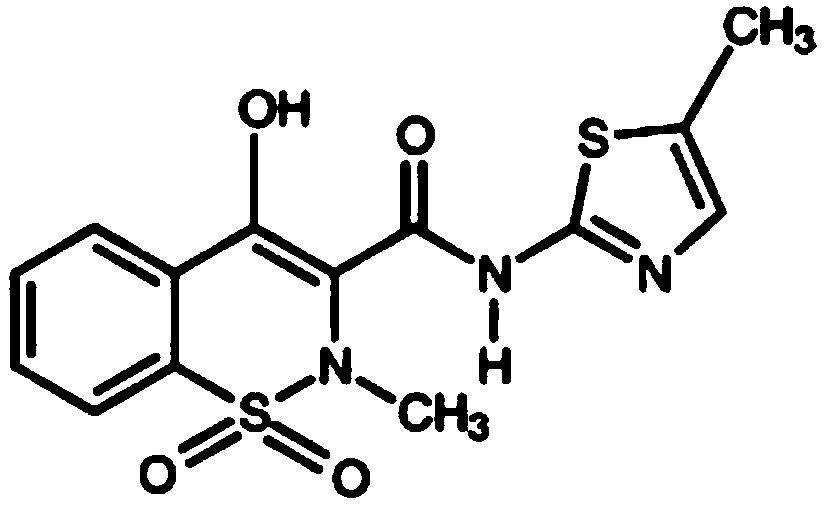
[0120] Example 1: Meloxicam 30mg IV Injection Formulation
[0121] The IV injection formulation is prepared as a ready-to-use formulation in a ready-to-use vial containing 30 mg meloxicam, povidone, sodium deoxycholate (deoxycholic acid), sucrose and water for injection in a total volume of 1 mL.
[0122] The placebo contained soybean oil, egg yolk phospholipids, glycerin, sodium fluorescein, sodium folate, disodium edetate, benzyl alcohol, polysorbate 80, dextrose, and water for injections. Hydrochloric acid and / or sodium hydroxide can be used for pH adjustment.
example 2
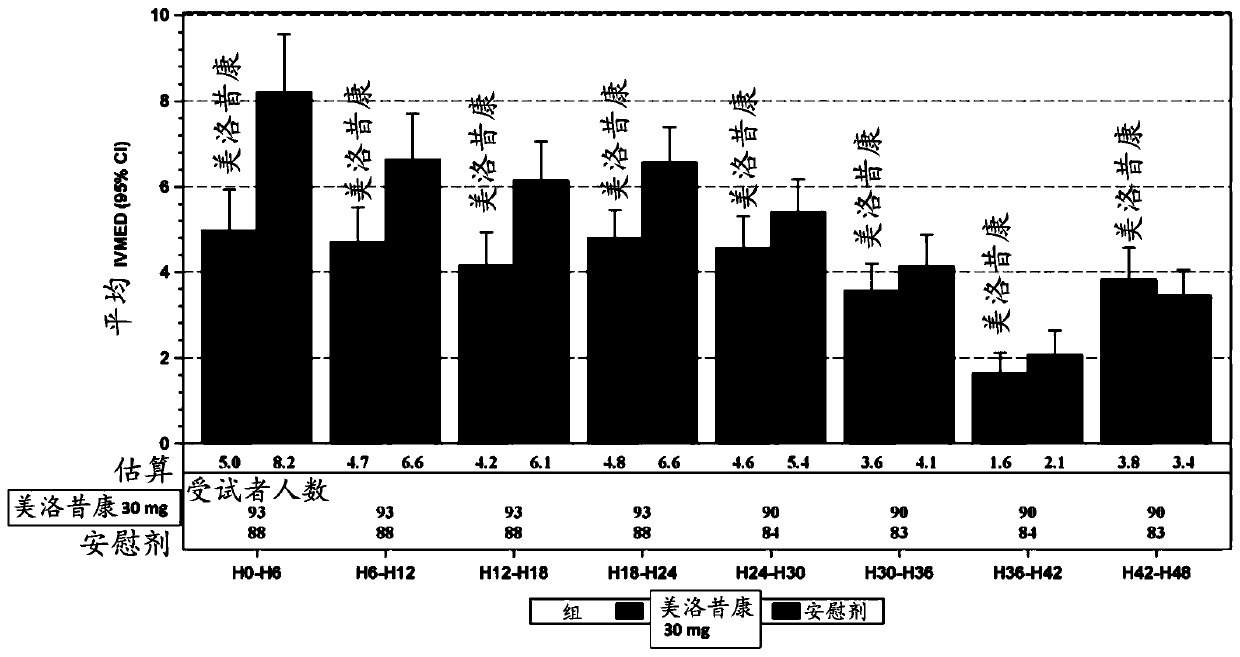
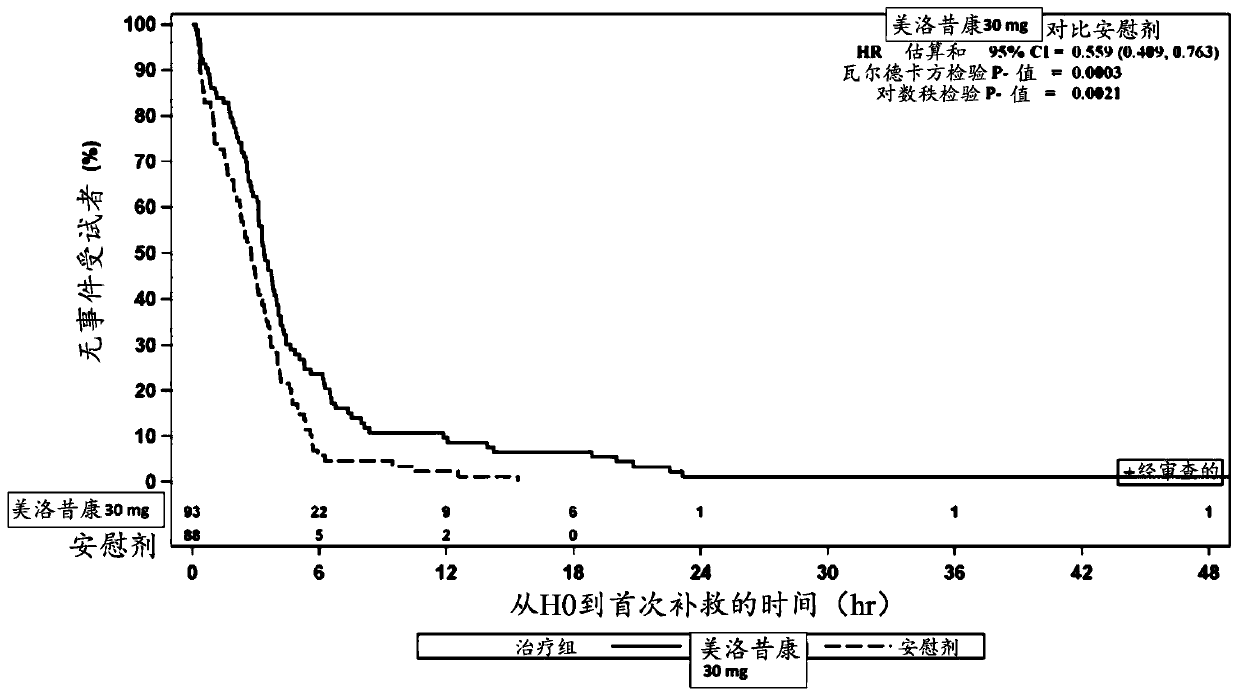
[0123] Example 2: Safety and efficacy of IV administration of meloxicam in combination with acetaminophen and gabapentin prior to colorectal surgery
[0124] An ongoing randomized, double-blind, placebo-controlled, multicenter study evaluating the safety of preoperative IV administration of meloxicam 30 mg in adult subjects undergoing open or laparoscopic colorectal surgery and effectiveness. The study cohort comprised approximately 50 subjects (1:1) randomized to 30 mg IV meloxicam or placebo. Adult subjects (ages 18 years (inclusive) to 80 years (inclusive)) scheduled to undergo colorectal surgery (expected to result in hospitalization for at least 48-72 hours) were screened for participation in the study at up to 20 study sites in the United States. Screening was performed within 28 days prior to IV administration of meloxicam. After signed informed consent, medical history, physical examination, laboratory tests, 12-lead electrocardiogram (ECG), pregnancy test, and vital...
example 3
[0131] Example 3: Safety and efficacy of IV administration of meloxicam in combination with acetaminophen and gabapentin before open unilateral total knee arthroplasty
[0132] method:A randomized, double-blind, placebo-controlled, multicenter study is ongoing to evaluate the safety of preoperative IV administration of meloxicam in adult subjects undergoing elective open unilateral total knee arthroplasty sex and effectiveness. Surgical procedures were performed in an inpatient hospital setting and were expected to result in a hospital stay of ≥24 hours. Each subject was screened within 28 days prior to surgery on Day 1. Prior to surgery, approximately 200 eligible subjects were randomized 1:1 to receive 30 mg IV meloxicam or placebo, administered as an intravenous (IV) bolus injection over ≤15 seconds.
[0133] Subjects received oral acetaminophen 650 mg and oral gabapentin 600 mg 30 to 90 minutes before the start of the planned surgical procedure. Subjects received appr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com