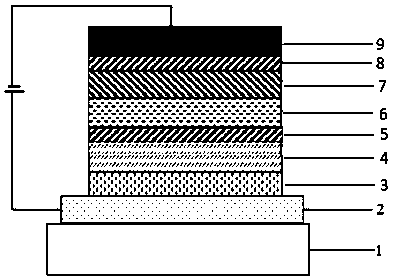
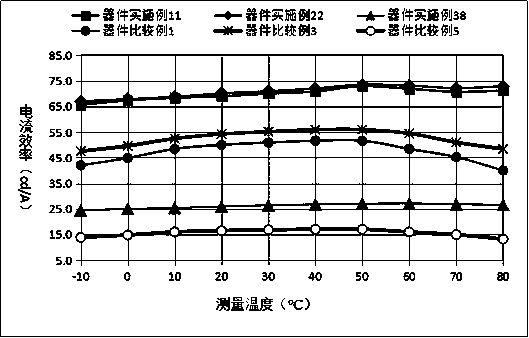
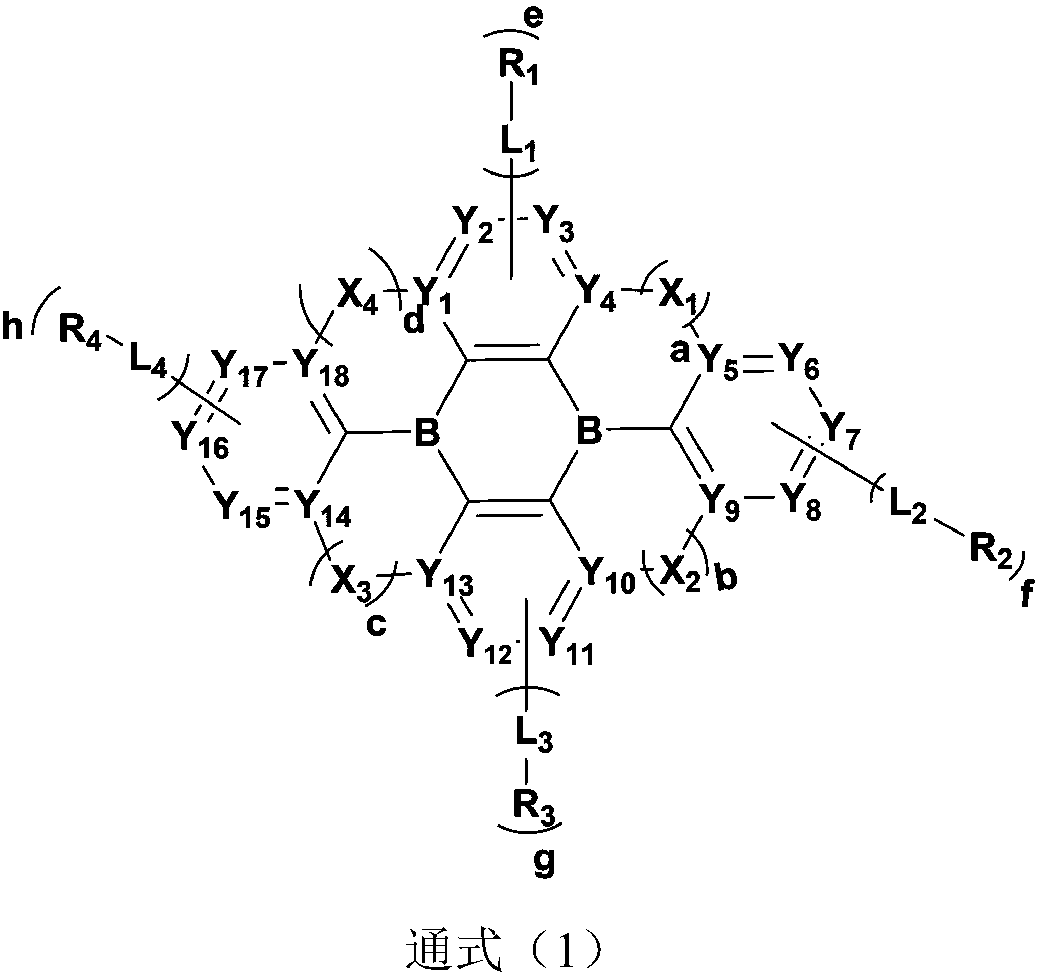
Organic compound with diboron as core, preparation method of organic compound and application of organic compound to organic light-emitting device
An organic compound and compound technology, which is applied in the fields of silicon-organic compounds, compounds containing Group 3/13 elements of the periodic table, and organic chemistry, etc. Transition rate, efficiency roll-off, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0140] Preparation of Compound 4
[0141]
[0142] 1) In a 250ml three-neck flask, under the protection of nitrogen, add 0.005mol of raw material 1 and 0.005mol of raw material 2, 0.015mol of tert-butyllithium, and 150ml of tert-butylbenzene, stir and mix, heat to 60°C, and stir for 2 hours; Then cool naturally to room temperature, dropwise add 0.015mol BBr 3 and 0.1mol diisopropylethylamine, stirred and reacted at room temperature for 1 hour, sampling and pointing the plate, showing that there was no raw material 1 remaining, and the reaction was complete; adding water and dichloromethane for extraction and liquid separation; taking the organic phase, adding anhydrous magnesium sulfate Remove water, filter, and the filtrate is subjected to decompression rotary evaporation (-0.09MPa, 25°C), and passes through a neutral silica gel column to obtain intermediate 1 with a HPLC purity of 99.5% and a yield of 65.7%; elemental analysis structure (molecular formula C 36 h 32 B ...
preparation Embodiment 2
[0145] Preparation of Compound 22
[0146]
[0147] Repeat the preparation process of Preparation Example 1, the difference is that raw material 4 is used instead of raw material 1, raw material 5 is used instead of raw material 2, intermediate 3 is prepared, intermediate 4 is obtained after bromination, intermediate 4 and raw material 6 are used for coupling reaction , the target compound 22 was obtained with a purity of 99.6% and a yield of 71.3%. Elemental analysis structure (molecular formula C 49 h 39 B 2 NO): theoretical value C, 86.62; H, 5.79; B, 3.18; N, 2.06; ESI-MS(m / z)(M + ): The theoretical value is 679.32, and the measured value is 679.71.
preparation Embodiment 3
[0148] Preparation of Compound 46
[0149]
[0150] Repeat the preparation process of Preparation Example 1, the difference is that raw material 7 is used instead of raw material 1, raw material 8 is used instead of raw material 2, intermediate 5 is prepared, intermediate 6 is obtained after bromination, intermediate 6 and raw material 9 are used for coupling reaction , the target compound 46 was obtained with a purity of 99.5% and a yield of 73.7%. Elemental analysis structure (molecular formula C 50 h 32 B 2 N 2 ): theoretical value C, 88.00; H, 4.73; B, 3.17; N, 4.10; test value: C, 88.01; H, 4.71; B, 3.15; N, 4.13. ESI-MS(m / z)(M + ): The theoretical value is 682.28, and the measured value is 682.55.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com