Lipopeptide compounds Totopotensamides and preparation method and application thereof
The technology of a compound, ppww50-totr1, is applied in the field of new antibiotic compounds, which can solve the problems such as the difficulty of making natural products into drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 1
[0228] Implementation Case 1: Construction of recombinant strain S.lividans TK64 / pCSG5549
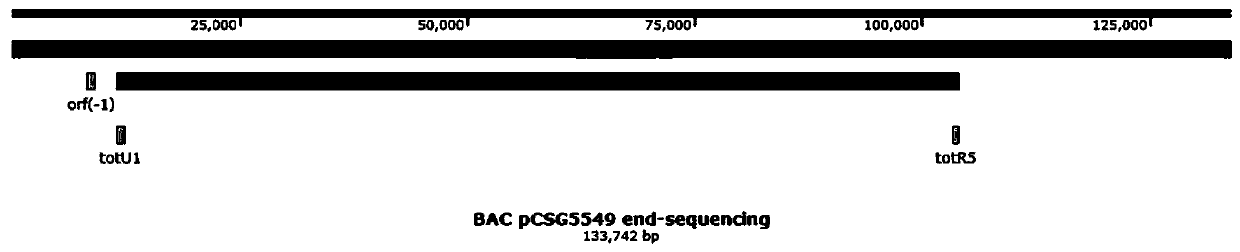
[0229] According to the tot biosynthetic gene cluster ( figure 1 ) of the upstream gene orf (-1) and downstream gene totR5 design primers orf (-1) testF / R and totR5testF / R (primer sequences are shown in Table 1), screened out in the BAC library of bacterial strain S.pactum SCSIO 02999 Clone pCSG5549, through terminal sequencing ( figure 2 ), it was confirmed that pCSG5549 contained all the genes of the totopotensamides biosynthetic gene cluster. The pCSG5549 was introduced into the heterologous host S. lividans TK64 by conjugative transfer of three parents. The process of conjugative transfer is described as follows: S.lividans TK64 was cultured by streaking on the SFM medium plate for 5-7 days, and the grown spores were collected in the TSB medium with a sterile cotton swab, and vortexed to disperse the spores; the hyphae were separated by filtration The spores and spores were susp...
Embodiment example 2
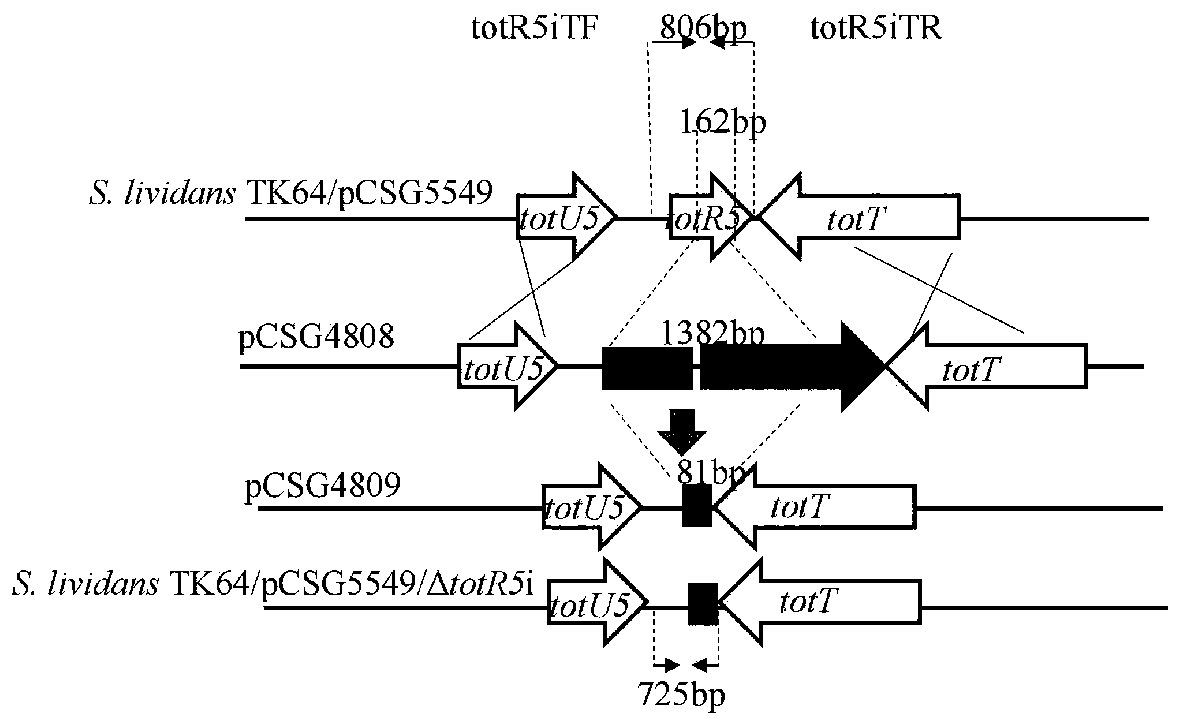
[0230] Example 2: Construction of recombinant strain S.lividans TK64 / pCSG5549 / ΔtotR5i / ΔtotR3i
[0231] By PCR-targeting method, the neo gene in cosmid pCSG4809 (totR5 gene was deleted in the same frame) was replaced with oriT / aadA fragment ( image 3 ). The specific instructions are as follows: (1) The plasmid pCSG4809 (see literature Chen, R.D., Zhang, Q.B., Tan, B., Zheng, L.J., Li, H.X., Zhu, Y.G., and Zhang, C.S. (2017) Genomemining and activation of a silent PKS / NRPS gene cluster direct the production of totopotensamides, Org. Lett.19, 5697-5700.) into Escherichia coli E.coli BW25113 / pIJ790 to obtain E.coli BW25113 / pIJ790 / pCSG4809, with 10mmol L -1 The L-arabinose induces the expression of the λ / red recombinant system, and prepares it as an electroporation-competent cell for use; (2) Digest the plasmid pIJ778 with endonucleases EcoR I and Hind III, and recover about 1.4kb of which contains the transfer origin and the DNA fragment of the spectinomycin resistance gene, a...
Embodiment example 3
[0233] Example 3: Construction of recombinant strain S.lividans TK64 / pCSG5549 / ΔtotR5i / ΔtotR3i / ΔtotP2i
[0234] Using the PCR targeting method, the gene knockout primer totP2-tarF / R (Table 1) was designed for cosmidpCSG4801 (see literature Chen, R.D., Zhang, Q.B., Tan, B., Zheng, L.J., Li, H.X., Zhu, Y.G., and Zhang, C.S. (2017) Genome mining and activation of a silent PKS / NRPS genecluster direct the production of totopotensamides, Org.Lett.19, 5697-5700.) totP2 was knocked out ( Figure 5), its construction method is consistent with the replacement of neo gene in cosmid pCSG4809 by oriT / aadA, and the plasmid pCSG5580 is obtained. The plasmid pCSG5580 is introduced into E.coli DH5α / BT340 and grown on the Kan plate at 28°C. Point-to-point screening on LB solid plates of Kan and Spc, for single clones that grow on LB solid plates containing Kan but not on LB solid plates containing Spc, use the verification primer totP2-TestF / R (Table 1) After verification, a single clone with s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com