Preparation method of moxifloxacin hydrochloride liposome
A technology of moxifloxacin hydrochloride lipid and moxifloxacin hydrochloride, which is applied in the field of preparation of moxifloxacin hydrochloride liposomes, can solve the problems of low encapsulation efficiency and drug loading, poor stability, etc., and achieve prolongation of drug retention time, Good stability and the effect of improving the encapsulation rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
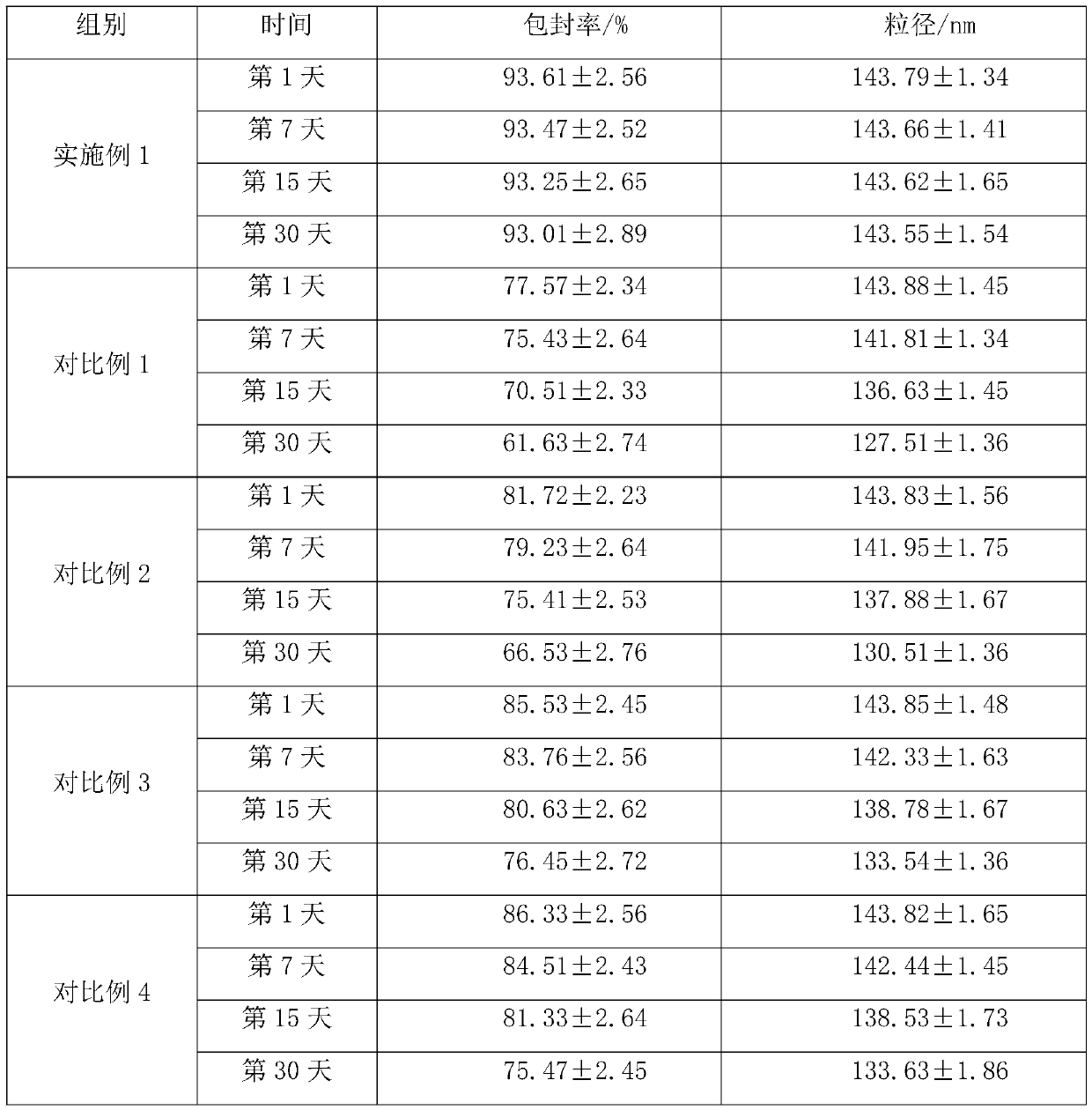
Embodiment 1
[0028] A preparation method of moxifloxacin hydrochloride liposomes, comprising the following steps:
[0029] S1, phospholipid and cholesterol are dissolved in chloroform, the mass concentration of phospholipid is 50mg / mL, the mass ratio of phospholipid and cholesterol is 5:1, add ammonium sulfate aqueous solution (the molar concentration of ammonium sulfate aqueous solution is 550mmol / L, ammonium sulfate The addition amount of aqueous solution is 2mL / 50mg phospholipid) carries out short-time ultrasonic treatment (power is 550W, time is 25s), then add xanthan gum and carboxymethyl chitosan, the mass ratio of xanthan gum and phospholipid is 1:4 , the mass ratio of carboxymethyl chitosan and phospholipids is 1:6, placed in a pressure of 800bar and a temperature of 65°C to carry out high-pressure homogenization treatment, high-pressure homogenization cycle 4 times, to obtain liposome protobody, lipid The liposomes were placed in an extruder, extruded whole particles with a 145nm ...
Embodiment 2
[0033] A preparation method of moxifloxacin hydrochloride liposomes, comprising the following steps:
[0034] S1, phospholipid and cholesterol are dissolved in chloroform, the mass concentration of phospholipid is 40mg / mL, the mass ratio of phospholipid and cholesterol is 4:1, add ammonium sulfate aqueous solution (the molar concentration of ammonium sulfate aqueous solution is 500mmol / L, ammonium sulfate The addition amount of aqueous solution is 1mL / 50mg phospholipid) carries out short-time ultrasonic treatment (power is 500W, time is 20s), then add xanthan gum and carboxymethyl chitosan, the mass ratio of xanthan gum and phospholipid is 1:3 , the mass ratio of carboxymethyl chitosan and phospholipids is 1:5, placed in a pressure of 700bar and a temperature of 60°C to carry out high-pressure homogenization treatment, high-pressure homogenization cycle 3 times, to obtain liposome protobody, lipid The liposomes were placed in an extruder, extruded whole particles with a 140nm ...
Embodiment 3
[0038] A preparation method of moxifloxacin hydrochloride liposomes, comprising the following steps:
[0039] S1, phospholipid and cholesterol are dissolved in chloroform, the mass concentration of phospholipid is 60mg / mL, the mass ratio of phospholipid and cholesterol is 6:1, add ammonium sulfate aqueous solution (the molar concentration of ammonium sulfate aqueous solution is 600mmol / L, ammonium sulfate The addition amount of aqueous solution is 3mL / 50mg phospholipid) carries out short-time ultrasonic treatment (power is 600W, time is 30s), then add xanthan gum and carboxymethyl chitosan, the mass ratio of xanthan gum and phospholipid is 1:5 , the mass ratio of carboxymethyl chitosan and phospholipids is 1:7, placed in a pressure of 900bar and a temperature of 70°C for high-pressure homogenization treatment, high-pressure homogenization cycle 5 times, to obtain liposome protobody, lipid The liposomes were placed in an extruder, extruded whole particles with a 150nm microporo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com