AP-2alpha monoclonal antibody and application to preparation of drugs for treating cervical cancers
An ap-2alpha, monoclonal antibody technology, applied in the direction of antibodies, anti-tumor drugs, drug combinations, etc., can solve the problem of less antibody research and achieve the effect of inhibiting cell proliferation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1 Screening and expression of AP-2alpha antigenic peptide fragments
[0033] According to the gene and amino acid sequence of AP-2alpha on NCBI, the inventor screened and obtained the AP-2alpha antigen fragment with high immunogenicity and isoelectric point through optimized screening of antigenic epitopes. Its amino acid sequence is shown as SEQ ID NO: As shown in 1, the isoelectric point pH 6.60 is suitable for prokaryotic expression, and the molecular weight of the protein is about 12.47KD.
[0034] SEQ ID NO: 1:
[0035] 1 QSQESGLLHT HRGLPHQLSG LDPRRDYRRH EDLLHGPHAL SSGLGDLSIH SLPHAIEEVPHVEDPGINIP DQTVIKKGPV
[0036] 81 SLSKSNSNAV SAIPINKDNL FGGVVNPNEV FCSVPG
[0037] After codon optimization, the corresponding nucleotide sequence was obtained, as shown in SEQ ID NO:2.
[0038] SEQ ID NO: 2:
[0039] 1 CAATCCCAGG AATCTGGTCT GCTGCATACT CATCGTGGTC TGCCTCATCA GCTGAGCGGTCTGGACCCTC
[0040] 71 GCCGTGATTA TCGTCGTCAC GAAGACCTGC TGCACGGTCC ACACGCACTG TCCAGCGGTC...
Embodiment 2
[0045] The preparation of embodiment 2 monoclonal hybridomas
[0046] 1. Immunization of mice:
[0047] The immunogen human AP-2alpha (prepared in Example 1) was emulsified with the volume of antigen and adjuvant at a ratio of 1:1, and Freund's complete adjuvant was used to emulsify the antigen for the first immunization, and the second immunization was started after 2 weeks , the antigen was emulsified with incomplete Freund's adjuvant, and injected subcutaneously at 2 points, the amount of antigen injected per mouse was 10 μg, and the volume injected at each injection point was 20 μL, a total of 10 mice.
[0048] Three days after the second immunization, blood was collected from the eyes of the mice, and a small amount of blood samples were taken for serum titer detection. The mice whose serum titer titer reached 1:150,000 were detected by indirect ELISA were strengthened. immunity.
[0049] 2. Preparation of feeder cells and myeloma cells
[0050] For the preparation of ...
Embodiment 3
[0065] Example 3 Detection of monoclonal antibody titer
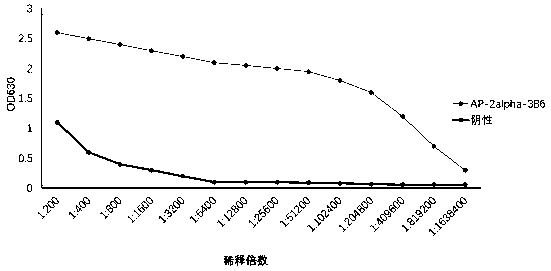
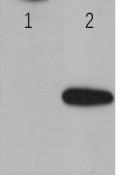
[0066] Use the indirect ELIS A method to detect the titer of the ascites obtained. The specific operation method is: use 28a-AP-2alpha purified protein and pET-28a empty carrier protein to coat the microtiter plate, and start to multiply the ascites at 1: 200 times Diluted as the primary antibody, and HRP-labeled goat anti-mouse IgG as the secondary antibody. When the OD630 value is greater than 1, the maximum dilution factor of the hybridoma cell ascites is the ELISA titer.
[0067] The results of indirect ELISA detection are shown in the figure ( figure 2 ), the ascites titer of AP-2alpha-3B6 antibody was 1:409600.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com