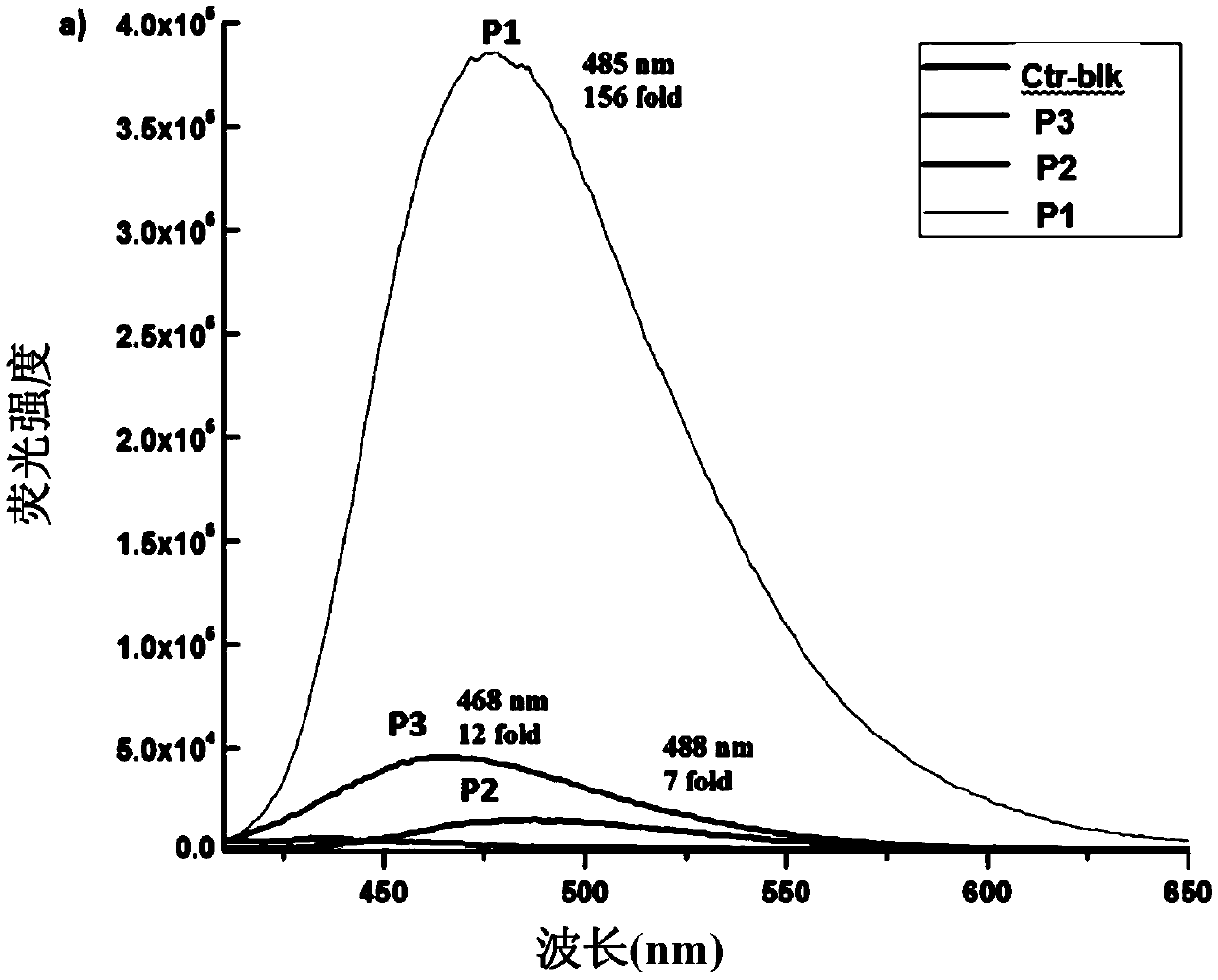
Fluorescent probe and preparation method thereof
A fluorescent probe, -et technology, applied in chemical instruments and methods, luminescent materials, organic chemistry, etc., can solve problems such as cumbersome operating procedures, troublesome probe purification, and difficult removal of fluorescent dyes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0103] An embodiment of the present invention also provides a method for preparing a fluorescent probe, comprising the following steps:
[0104] 1) Put nitroso or hydroxyamino substituted aromatic compounds and 1,3-diene compounds in a mixed solvent of acetonitrile and water in a molar ratio of 1:1 to 10 (volume ratio, acetonitrile: water = 1:1 to 6), react at 0-60°C for 15 minutes to 4 hours;
[0105] 2) The mixed solvent of acetonitrile and water was removed in vacuo, and then separated by column chromatography (H60 silica gel column, ethyl acetate:n-hexane=1:1-10) to obtain fluorescent probe molecules.
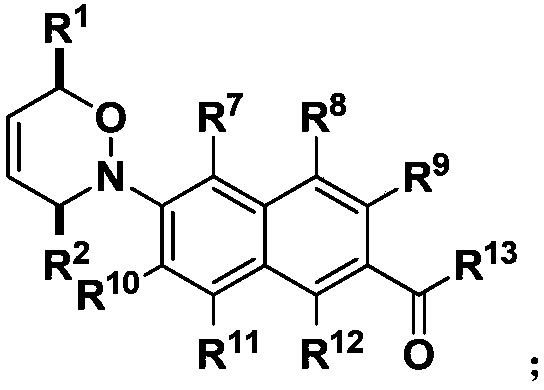
[0106] The obtained fluorescent probe molecules are the aforementioned first fluorescent probe molecules or second fluorescent probe molecules.
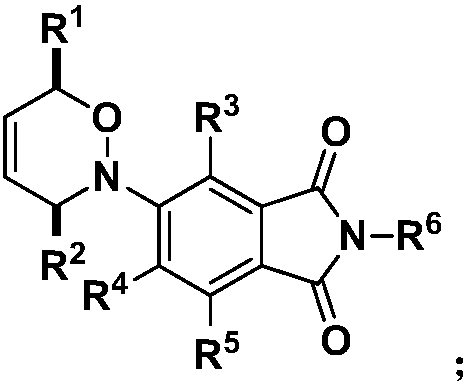
[0107] In step 1), the molecular structural formula of the nitroso-substituted aromatic compound is:
[0108]
[0109] R 3 -H (hydrogen), -Me (methyl), -Et (ethyl), -Pr (propyl), -iPr (isopropyl), -Bu (butyl), -tBu (tert-but...
Embodiment 1
[0141] Fluorescent probe molecule Probe 1 (referred to as P 1, [English full name: Benzyl3-(2-methyl-1,3-dioxoisoindolin-5-yl)-2-oxa-3-azaspiro[bicyclo[2.2.1]hept[5 ]ene-7,4'-piperidine]-1'-carboxylate]) is as follows:
[0142]
[0143] The synthetic method of fluorescent probe molecule P1:
[0144] Dissolve 53.8mg (0.2mmol) benzyl 8-azaspiro[4.5]deca-1,3-diene-8-carboxylate and 41.8mg (0.22mmol) 2-methyl-5-nitrosoisoindoline-1,3-dione in acetonitrile / water (2.0 ~ 4.0mL). Stir at 25°C. Then the reaction solvent was concentrated and purified by column chromatography to obtain the corresponding fluorescent probe molecule P1 (H60 silica gel column, ethyl acetate:n-hexane=1:2).
[0145] NMR spectroscopy analysis:
[0146] 1 H NMR (400MHz, CDCl 3 )δ7.61-7.63(d,J=8Hz,1H),7.32-7.37(m,6H),7.11-7.14(dd,J=4Hz,8Hz,1H),6.32-6.35(m,1H),5.94 -5.97(m,1H),5.140(s,2H),4.81-4.83(m,1H),4.73-4.75(m,1H),3.56-3.58(m,2H),3.40-3.44(m,2H) ,3.12(s,3H),1.86-1.98(m,2H),1.58-1.60(m,2H); 13 C N...
Embodiment 2
[0150] Fluorescent probe molecules are referred to as P1 structural formula as follows:
[0151]
[0152] The synthetic method of fluorescent probe molecule P1:
[0153]Dissolve 53.8mg (0.2mmol) benzyl 8-azaspiro[4.5]deca-1,3-diene-8-carboxylate and 42.2mg (0.22mmol) 5-(hydroxyamino)-2-methylisoindoline-1,3-dione in acetonitrile / water (2.0-4.0mL). Stir at 25°C. Then the reaction solvent was concentrated and purified by column chromatography to obtain the corresponding fluorescent probe molecule P1 (H60 silica gel column, ethyl acetate:n-hexane=1:2).
[0154] NMR spectroscopy analysis:
[0155] 1 H NMR (400MHz, CDCl 3 )δ7.61-7.63(d,J=8Hz,1H),7.32-7.37(m,6H),7.11-7.14(dd,J=4Hz,8Hz,1H),6.32-6.35(m,1H),5.94 -5.97(m,1H),5.140(s,2H),4.81-4.83(m,1H),4.73-4.75(m,1H),3.56-3.58(m,2H),3.40-3.44(m,2H) ,3.12(s,3H),1.86-1.98(m,2H),1.58-1.60(m,2H); 13 C NMR (100MHz, CDCl 3 )δ168.55, 168.34, 156.37, 136.73, 133.67, 132.52, 131.71, 128.53, 128.07, 124.21, 123.96, 110.85, 99.98, 85...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com