5-fluorouracil-caffeic acid eutectic crystal and preparation method thereof
A technology of fluorouracil and caffeic acid, which is applied in the pharmaceutical co-crystal of 5-fluorouracil and caffeic acid and its preparation field, can solve the problems of low bioavailability, poor physical and chemical properties, complex process technology, etc., and achieve high yield and purity, The effect of mild reaction conditions and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
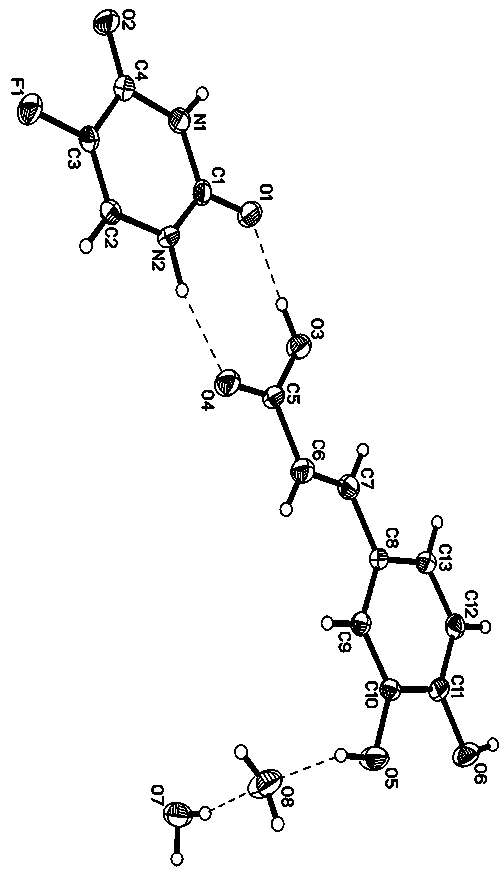
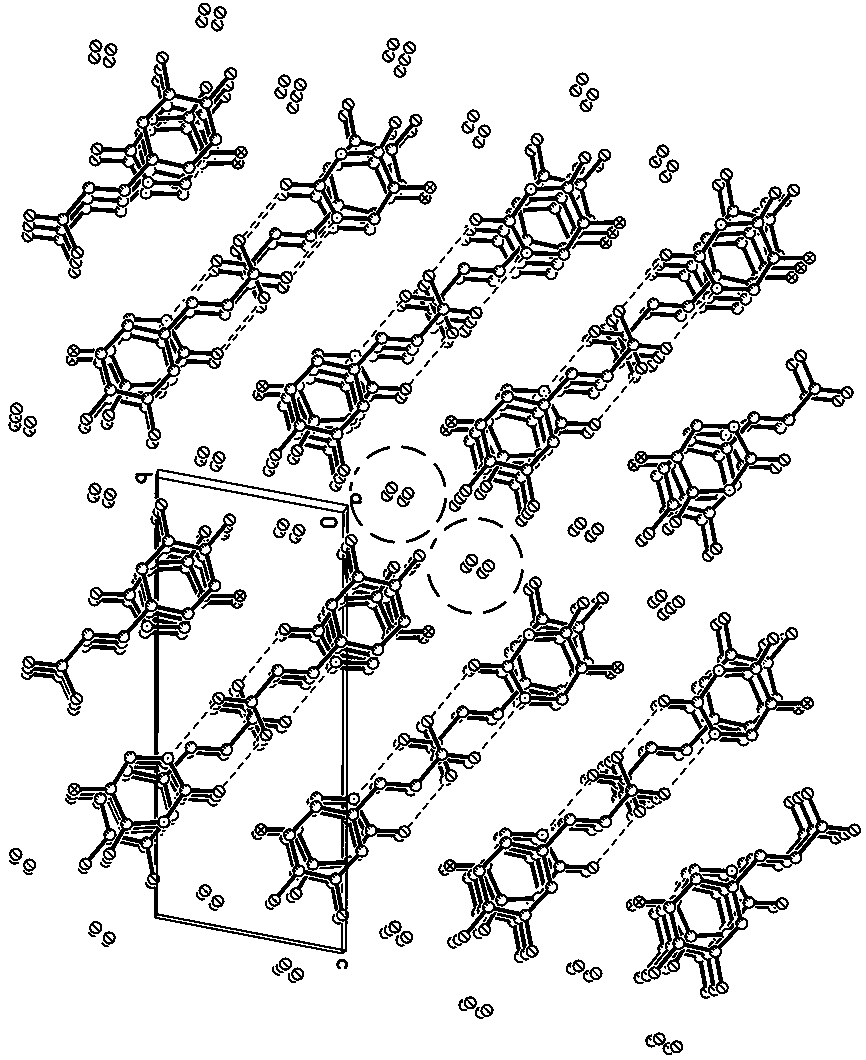
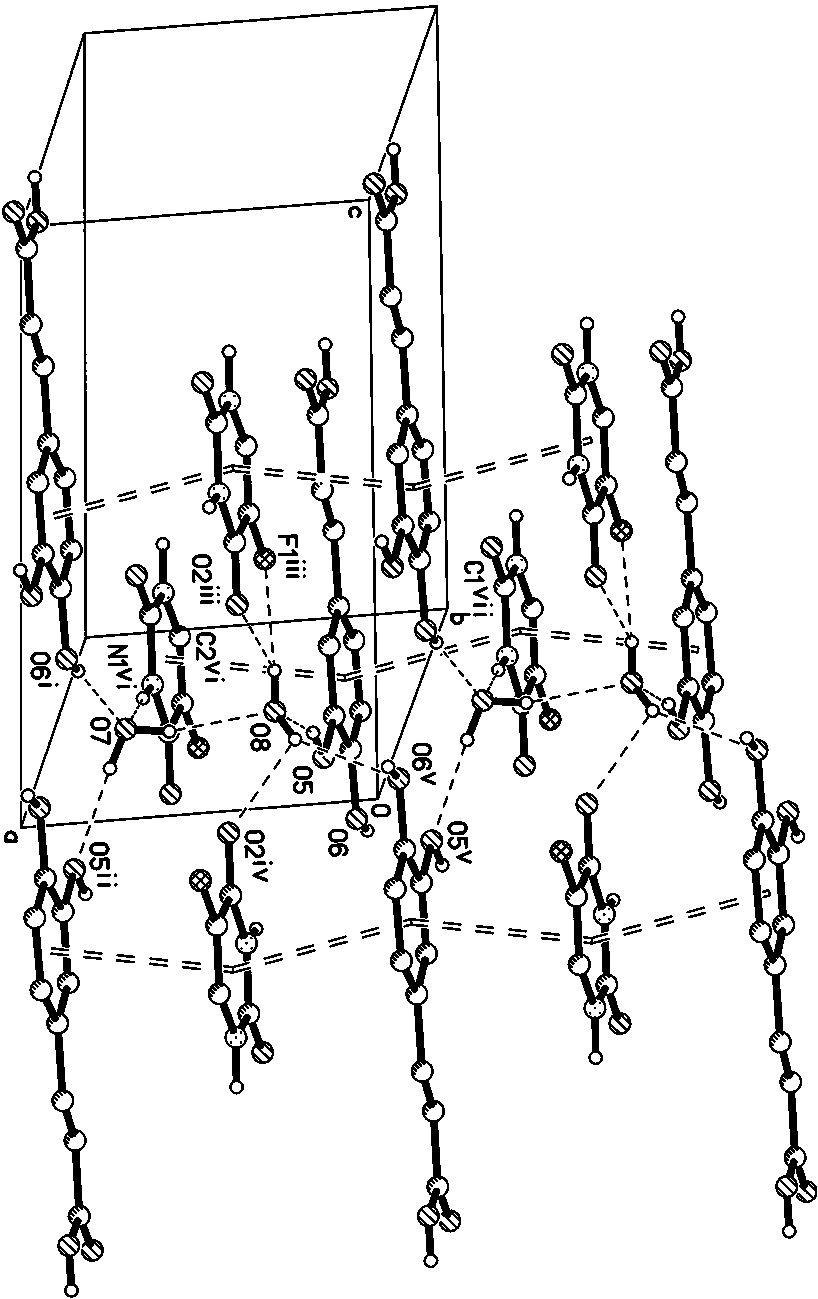
[0021] Specific implementation mode one: the molecular formula of the 5-fluorouracil-caffeic acid co-crystal in this embodiment is [C 4 h 3 N 2 o 2 F·C 9 h 8 o 4 2H 2 O], a 5-fluorouracil molecule, a caffeic acid molecule and two water molecules constitute the basic structural unit, the 5-fluorouracil-caffeic acid cocrystal belongs to the triclinic system, and the space group is P -1, the unit cell parameters are: a = 6.9299 Å, b = 6.9584 Å, c = 15.946 Å, α = 99.753°, β = 96.384°, γ = 98.812°. Its PXRD characteristic diffraction peaks appear at 11.36°, 13.1°, 15.64°, 19.48°, 26.08°, 28.12°.
[0022] The 5-fluorouracil-caffeic acid co-crystal described in this embodiment consists of one 5-fluorouracil molecule, one caffeic acid molecule and two water molecules bonded together through supramolecular interactions such as hydrogen bonds. Such as figure 1 As shown, the co-crystal is a complex formed by 5-fluorouracil and caffeic acid at a molar ratio of 1:1, and contains t...
specific Embodiment approach 2
[0023] Specific embodiment two: the preparation method of the present embodiment 5-fluorouracil-caffeic acid co-crystal is implemented according to the following steps:
[0024] Put 5-fluorouracil raw material and caffeic acid in a round-bottomed flask at a molar ratio of 1:1, add methanol and water (1:2, v / v) to the mixed powder, stir for 4-6 h and then filter, the filtrate After standing for 2-5 days, the solid phase was collected and dried in vacuum to obtain 5-fluorouracil-caffeic acid co-crystal.
specific Embodiment approach 3
[0025]Embodiment 3: This embodiment differs from Embodiment 2 in that the solid-to-liquid ratio of the mixture powder to the solvent in the system is 100 mg: (18-24) mL. Other steps and parameters are the same as in the second embodiment.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com