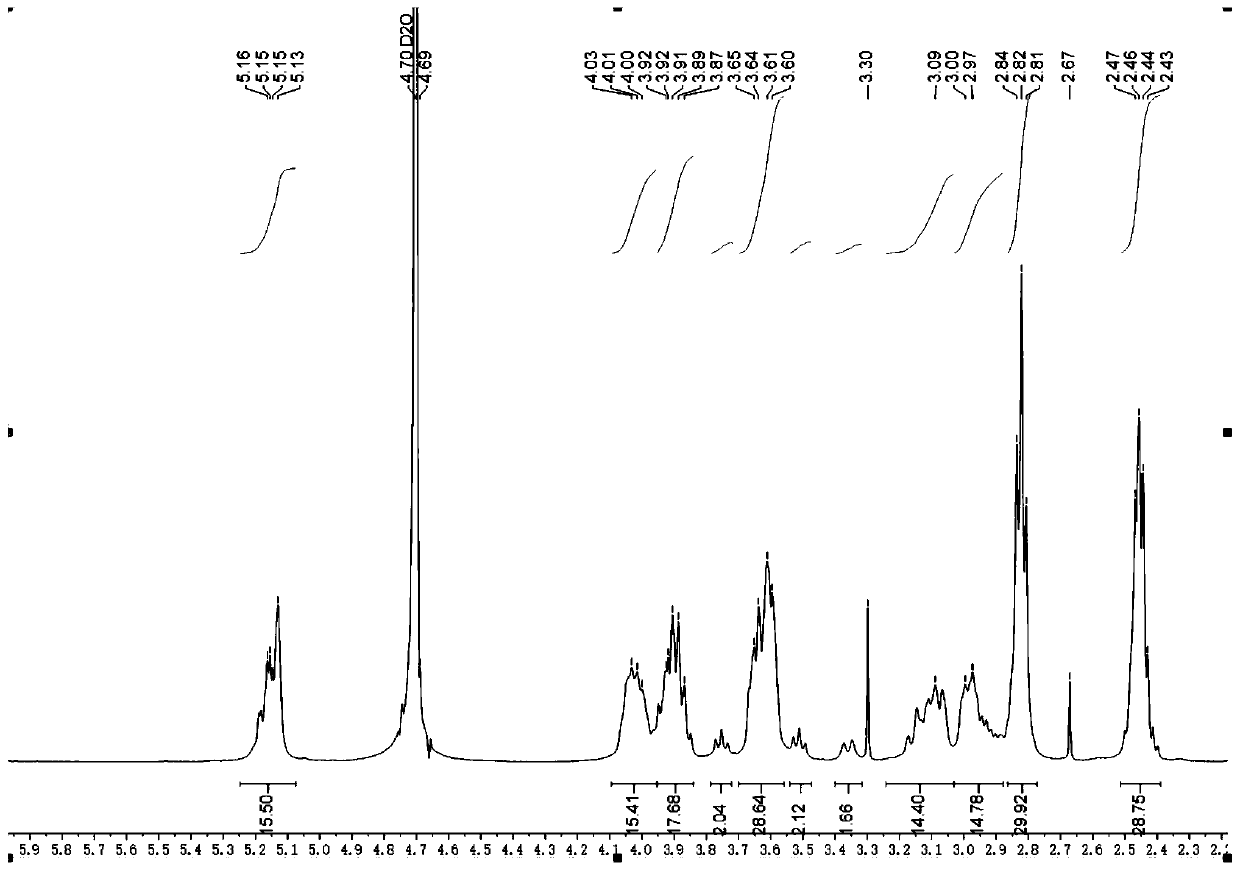
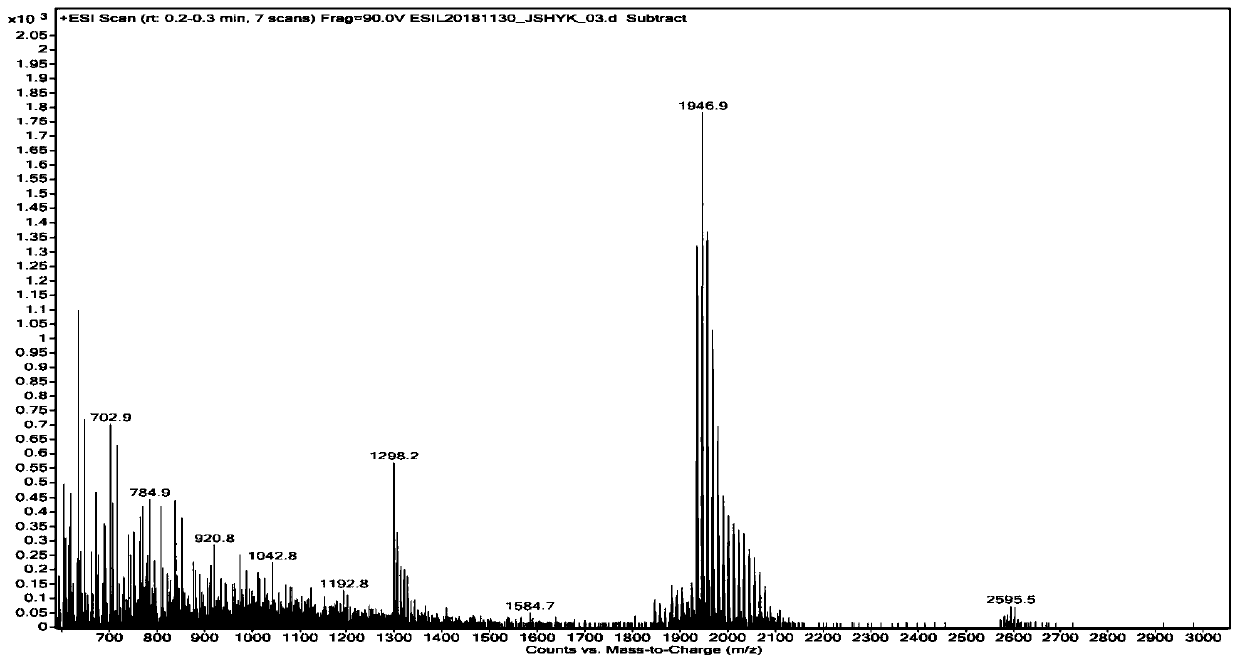
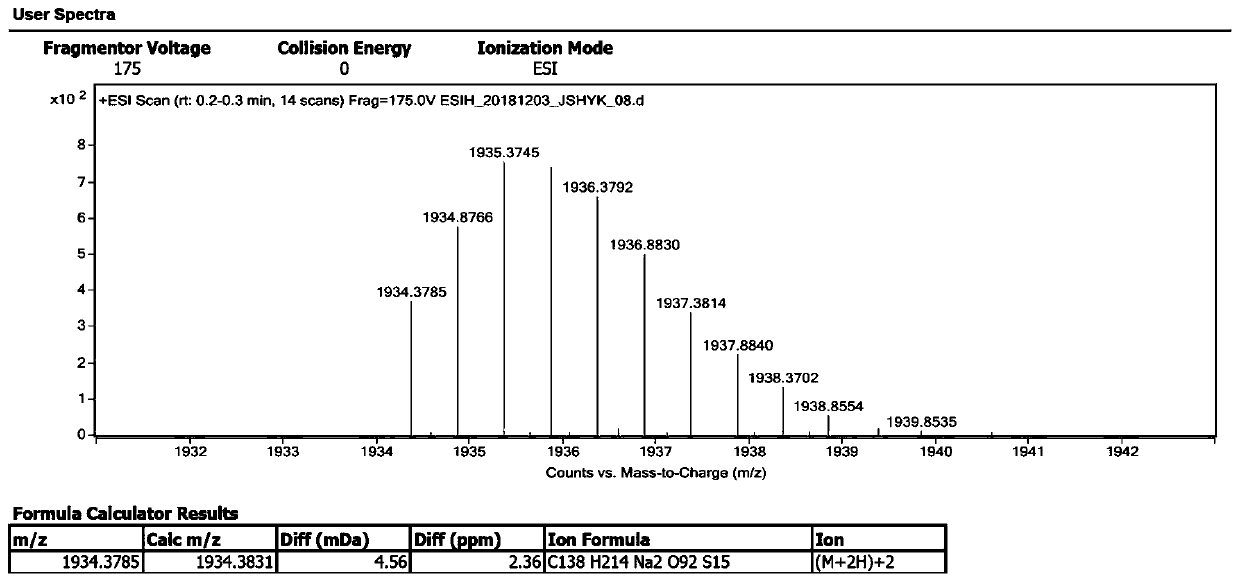
Synthesis method of sugammadex sodium dimer impurity
A technology of sugammadex sodium and a synthesis method, which is applied in the field of chemical substance synthesis and preparation, can solve problems such as poor qualitative and quantitative accuracy, and achieve the effects of improving quality, improving accuracy and reducing risks.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] The examples are used to illustrate the present invention, but should not be construed as limiting the present invention.
[0040] Preparation of Intermediate 3:
[0041] Add octa-(6-bromo-6-deoxy)-γ-cyclodextrin (40g, 22.2mmol) and 140mL DMF to a 250mL reaction flask, stir to dissolve. Add sodium sulfide nonahydrate (2.67g, 11.1mmol) and react at 20°C for 24 hours. Filter to remove undissolved sodium sulfide nine water. Add 140 mL of DMF to the filtrate to dilute, add 450 mL of methyl isobutyl ketone dropwise, stir for half an hour after dropping, and filter to obtain a crude product. The crude product was repeatedly purified by DMF / methyl isobutyl ketone 3 to 5 times to remove the raw material octa-(6-bromo-6-deoxy)-γ-cyclodextrin. The crude product was purified by DMF / MeOH / water repeatedly 5 times to remove intramolecular thioether by-products. After vacuum drying at 45°C, 2.2 g of Intermediate 3 was finally obtained as a light gray solid.
Embodiment 2
[0043] The examples are used to illustrate the present invention, but should not be construed as limiting the present invention.
[0044] Preparation of compound 1:
[0045] Add sodium hydroxide (1.47g, 36.6mmol) and 6.4mL of purified water to a 100mL three-necked flask, stir and dissolve, then fully replace with nitrogen for protection, cool down to 0-10°C and add 3-mercaptopropionic acid (1.95g, 18.3mmol) dropwise ). After the drop, the temperature was raised to 40°C, and 2.12g (0.63mmol) of intermediate 3 in 16mL of DMSO was slowly added, and the reaction was carried out at 45-50°C for four hours after the addition. After adding 11.2 mL of water, the reaction solution became clear. After maintaining the temperature for half an hour, add 4.2 mL of DMSO, cool down to 0-5°C for crystallization and filtration, rinse the filter cake with absolute ethanol, and dry it in vacuum at 40°C to obtain 2.2 g of crude product. The crude product was recrystallized from water / methanol an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com