Method for preparing sterol derivative through Burkholderia conversion and application
A technology of Burkholderia and Krkholderia, applied in the field of Burkholderia transformation and preparation of sterol derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
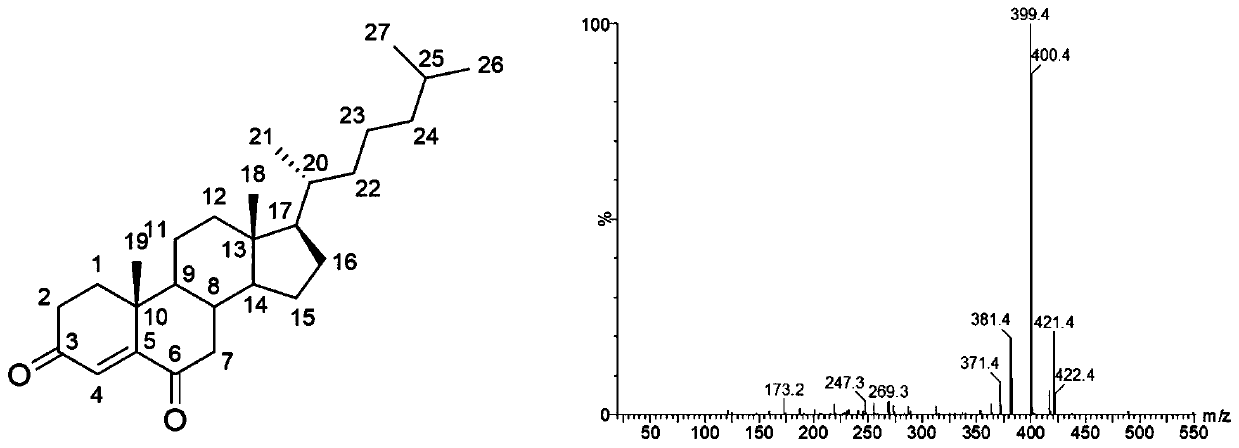
[0035]Example 1: α-cyclodextrin system B.cepacia converts cholesterol to prepare cholest-4-ene-3,6-dione
[0036] (1) Slant culture: Take Burkholderia ZWS15 stored in a glycerol tube at -40°C, inoculate 30 μL into LB solid medium, place it in a constant temperature incubator at 37°C, and cultivate for 24 hours until a single colony grows;
[0037] (2) Seed culture: Pick a single colony from the strain cultivated in step (1) under sterile conditions and inoculate it in 50 mL LB liquid medium, and culture it in a shaker at 37°C with a rotation speed of 200 rpm for 9-13 hours to OD 600 5.0 to 6.0.
[0038] (3) B. cepacia conversion of cholesterol: the seed solution cultivated in step (2) was inserted into the transformation medium at an inoculum size of 5% by volume, and cultured at 30° C. and 200 rpm for 48 hours. The group without cyclodextrin or its derivatives was used as the control.
[0039] (4) TLC separation and extraction of the conversion product of cholesterol: the ...
Embodiment 2
[0045] Example 2: β-cyclodextrin system B.cepacia converts cholesterol to prepare cholest-4-ene-3,6-dione
[0046] See embodiment 1 for the specific embodiment.
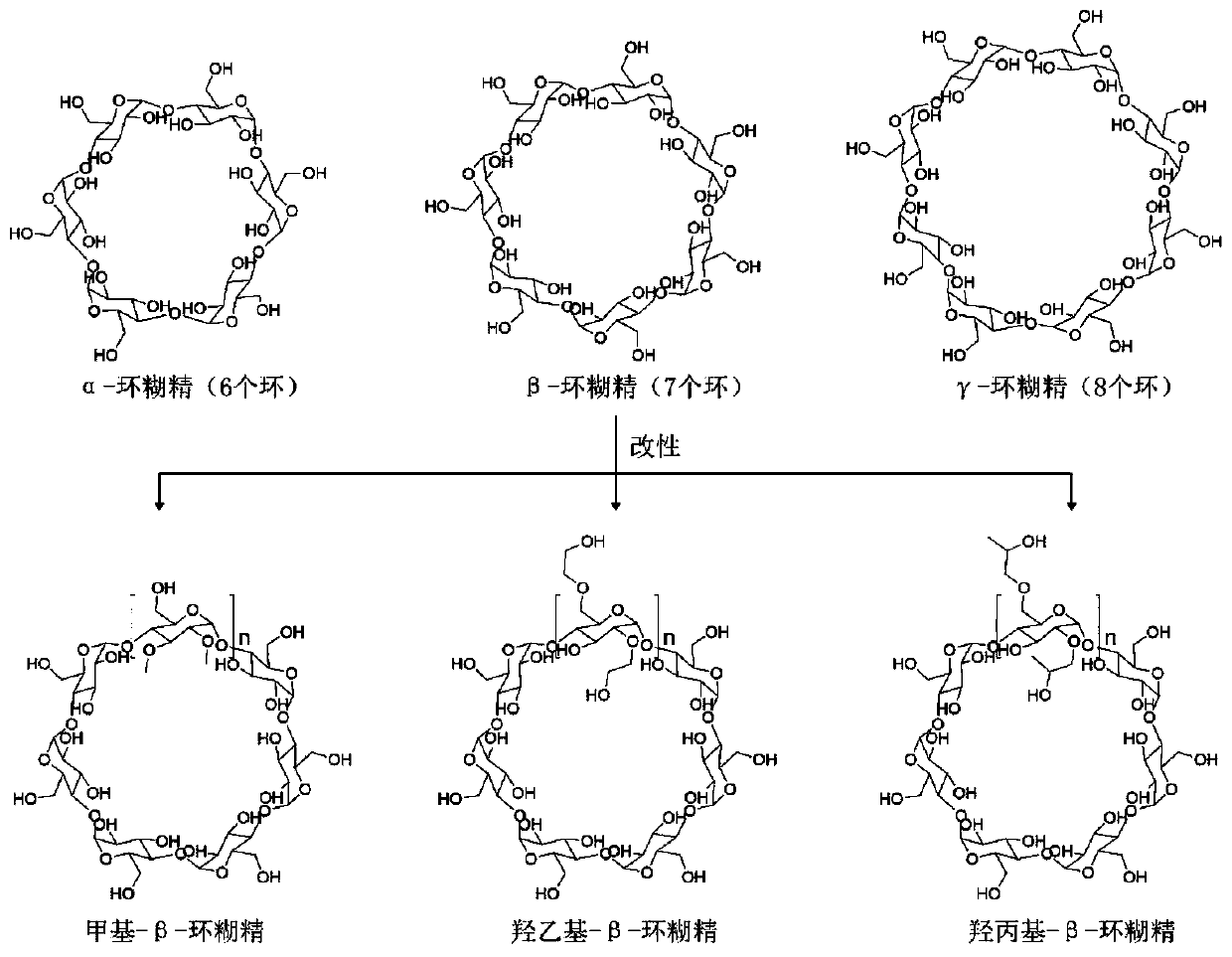
[0047] The amount of fixed cholesterol in the transformation medium was 2g / L, that is, 5.18mM; the molar ratio of β-cyclodextrin to cholesterol was 1:2, 1:1 and 2:1. The two were pretreated with 60W ultrasonic for 10-15min and then added to the transformation medium.
[0048] Analysis and detection: When the molar ratio of β-cyclodextrin and cholesterol is 1:2, 1:1 and 2:1, the molar conversion rate of cholest-4-ene-3,6-dione is 0.38% respectively , 0.36% and 0.42%. The conversion rate of the control group under the same conditions was 0.2%.
Embodiment 3
[0049] Example 3: γ-cyclodextrin system B.cepacia converts cholesterol to prepare cholest-4-ene-3,6-dione
[0050] See embodiment 1 for the specific embodiment.
[0051] The amount of fixed cholesterol in the transformation medium was 5.18mM; the molar ratio of γ-cyclodextrin to cholesterol was 1:2, 1:1 and 2:1. The two were pretreated with 60W ultrasonic for 10-15min and then added to the transformation medium.
[0052] Analysis and detection: when the molar ratio of γ-cyclodextrin to cholesterol is 1:2, 1:1 and 2:1, the molar conversion rate of cholest-4-ene-3,6-dione is 0.51% respectively , 0.35% and 0.26%. The conversion rate of the control group under the same conditions was 0.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com