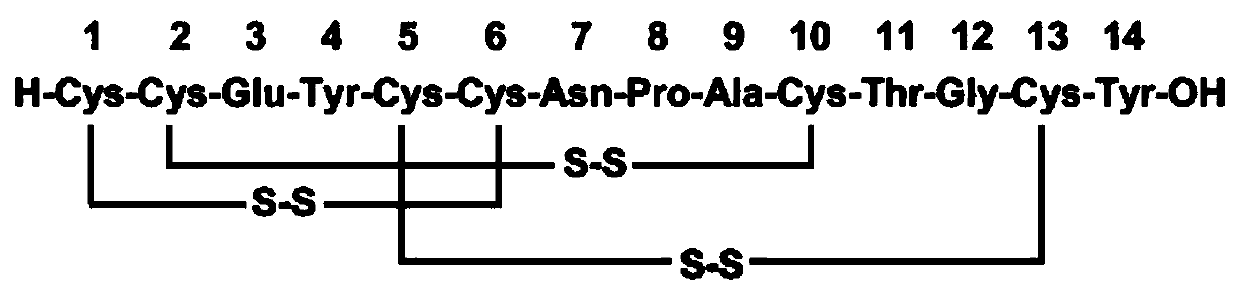
Method for preparing linaclotide through solid-liquid combination
A technology for linaclotide and lotide, which is applied in the field of preparing linaclotide by solid-liquid combination, can solve the problems of low total yield, low purity of linear crude peptide, inability to solve the purity of linear crude peptide, etc. Conducive to the effect of synthesis scale and production cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1: Synthesis of Fmoc-Gly-Cys(tButhio)-OH
[0048] Accurately weigh 2.09Kg (10mol) of H-Cys(tButio)-OH into a 50L reaction kettle, add 12L of 10% sodium carbonate aqueous solution by mass percentage, and dissolve under stirring; after the solution is clear, add Fmoc-Gly- OSu 3.94kg (10mol) / 12L tetrahydrofuran solution, stirred and reacted, TLC monitored the end point; after concentration under reduced pressure, acid adjustment, ethyl acetate extraction, drying, and crystallization, the dipeptide monomer Fmoc-Gly-Ser with a purity of 99.1% was obtained (tBu)-OH 3.86kg, yield 76.2%.
Embodiment 2
[0049] Embodiment 2: the synthesis of Fmoc-Pro-Ala-OH
[0050] Accurately weigh 1.07kg (12mol) of alanine in a 50L reaction kettle, add 12L of 10% sodium carbonate aqueous solution by mass percentage, and dissolve under stirring; after the solution is clear, add Fmoc-Pro-OSu 4.34kg (1mol ) / 12L tetrahydrofuran solution, stirred and reacted, and the end point was monitored by TLC; after concentration under reduced pressure, acid adjustment, ethyl acetate extraction, drying, and crystallization, the dipeptide monomer Fmoc-Pro-Ala-OH with a purity of 99.2% was obtained 3.27kg, Yield 78.2%.
Embodiment 3
[0051] Example 3: Preparation of linear linaclotide resin
[0052] Weigh 75 g (50 mmol) of Fmoc-Tyr(tBu)-Wang resin with a degree of substitution of 0.67 mmol / g and place it in a peptide resin synthesis reactor, add 700 mL of DCM to swell for 2 h. After the swelling is completed, wash with DMF three times, 600 mL each time, and then add 600 mL of 20% by volume piperidine / DMF solution for deprotection twice, for 10 min and 10 min respectively. After the deprotection was completed, the resin was washed 6 times with DMF, 600 mL each time. Weigh 49g of Fmoc-Gly-Cys(tButhio)-OH, 13.5g of HOBt and dissolve in 300mL of DMF, add 17mL of DIC to activate, add the solution into the reactor, react for 2h, and monitor the reaction end point by Kaiser test. After the reaction, the resin was washed 5 times with DMF, and then the next protected amino acid was deprotected and coupled. Repeat the above steps, followed by Fmoc-Thr(tBu)-OH, Fmoc-Cys(tButio)-OH, Fmoc-Pro-Ala-OH, Fmoc-Asn(Trt)-OH...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com