Targeted gene integration of nk inhibitors genes for improved immune cells therapy
A technology of immune cells and inhibitors, applied in the field of β2m locus insertion, sequence-specific endonuclease reagents and donor DNA vectors, can solve the problems of reducing immune response and lifespan, cell exhaustion, etc., and achieve transcriptional activity. Effects reduced by immune cell activation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0468] Example 1: AAV-driven homologous recombination at different loci in human primary T cells under the control of an endogenous promoter knocked out of an endogenous gene.
[0469] Introduction
[0470] Sequence-specific endonuclease reagents such as (Cellectis, 8 rue de la Croix Jarry, 75013 PARIS) enables site-specific induction of double-strand breaks (DSBs) at desired loci in the genome. Repair of DSBs by cellular enzymes occurs mainly through two pathways: non-homologous end joining (NHEJ) and homology-mediated repair (HDR). ADR uses homologous DNA fragments (template DNA), repairs DSBs by recombination, and can be used to introduce any gene sequence contained in the template DNA. As shown therein, recombinant adeno-associated virus (rAAV) with engineered nucleases such as The template DNA is delivered together to introduce site-specific DSBs.
[0471] Integrated Matrix Design
[0472] 1.1 Insertion of an apoptotic CAR at an upregulated locus knocked out of the...
Embodiment 2
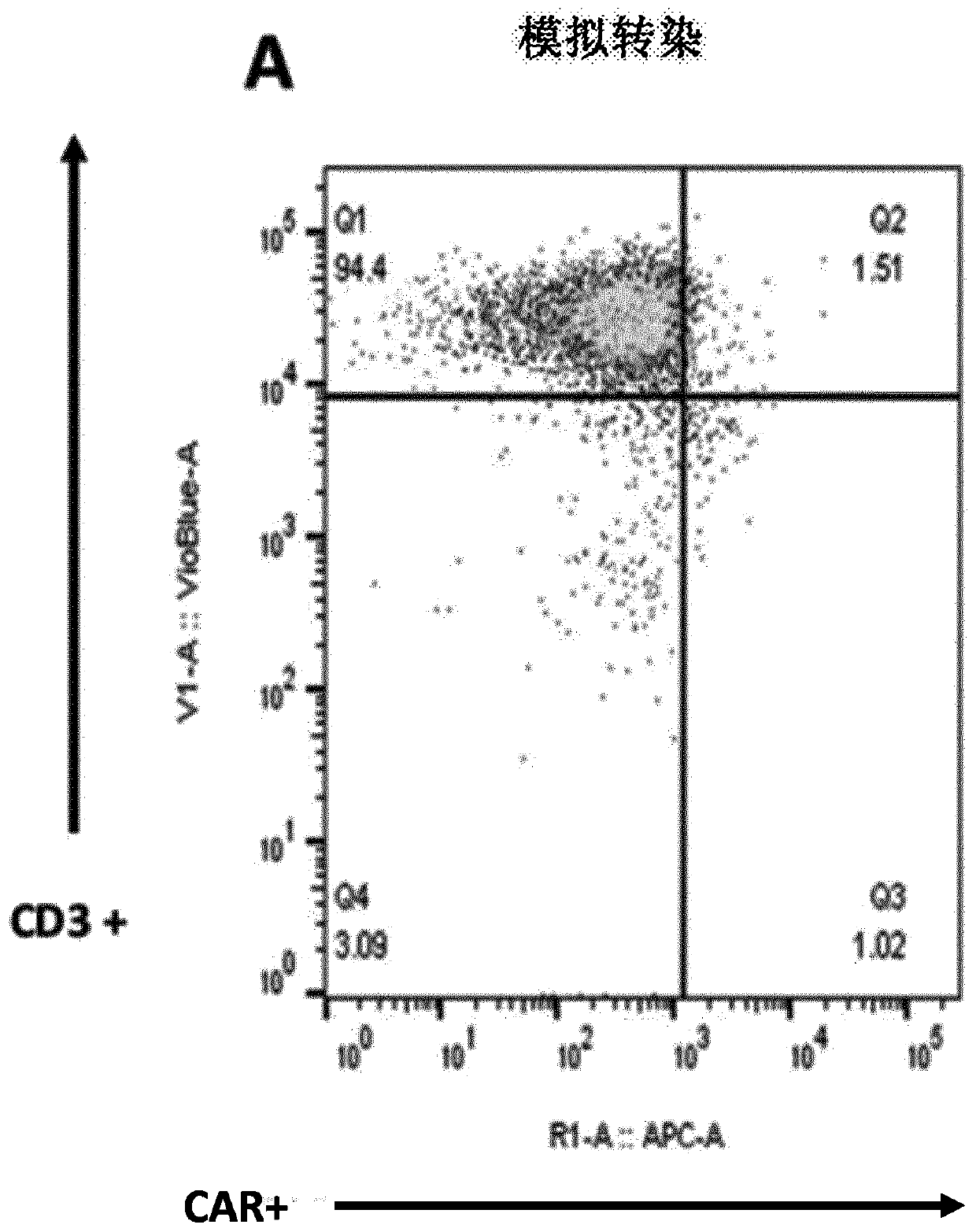
[0492] Example 2: In T cells - Mediated dual-targeted integration of IL-15 and CAR-encoded substrates
[0493] Material
[0494]X-vivo-15 was obtained from Lonza (cat#BE04-418Q), IL-2 was obtained from Miltenyi Biotech (cat#130-097-748), human serum AB was obtained from Seralab (cat#GEM-100-318), human T activator CD3 / CD28 was obtained from LifeTechnology (cat#11132D), QBEND10-APC was obtained from R&D Systems (cat#FAB7227A), vioblue-labeled anti-CD3, PE-labeled anti-LNGFR, APC-labeled anti-CD25 and PE - Labeled anti-PD1 was obtained from Miltenyi (cat#130-094-363, 130-112-790, 130-109-021 and 130-104-892, respectively), 48-well processing plate (CytoOne, cat#CC7682-7548 ), the human IL-15 Quantikine ELISA kit was obtained from R&D systems (cat#_S1500), and ONE-Glo was obtained from Promega (cat#E6110). AAV6 batches containing different matrices were obtained from Virovek, PBMC cells were obtained from Allcells (cat#PB004F) and Raji cells were obtained after transduction o...
Embodiment 3
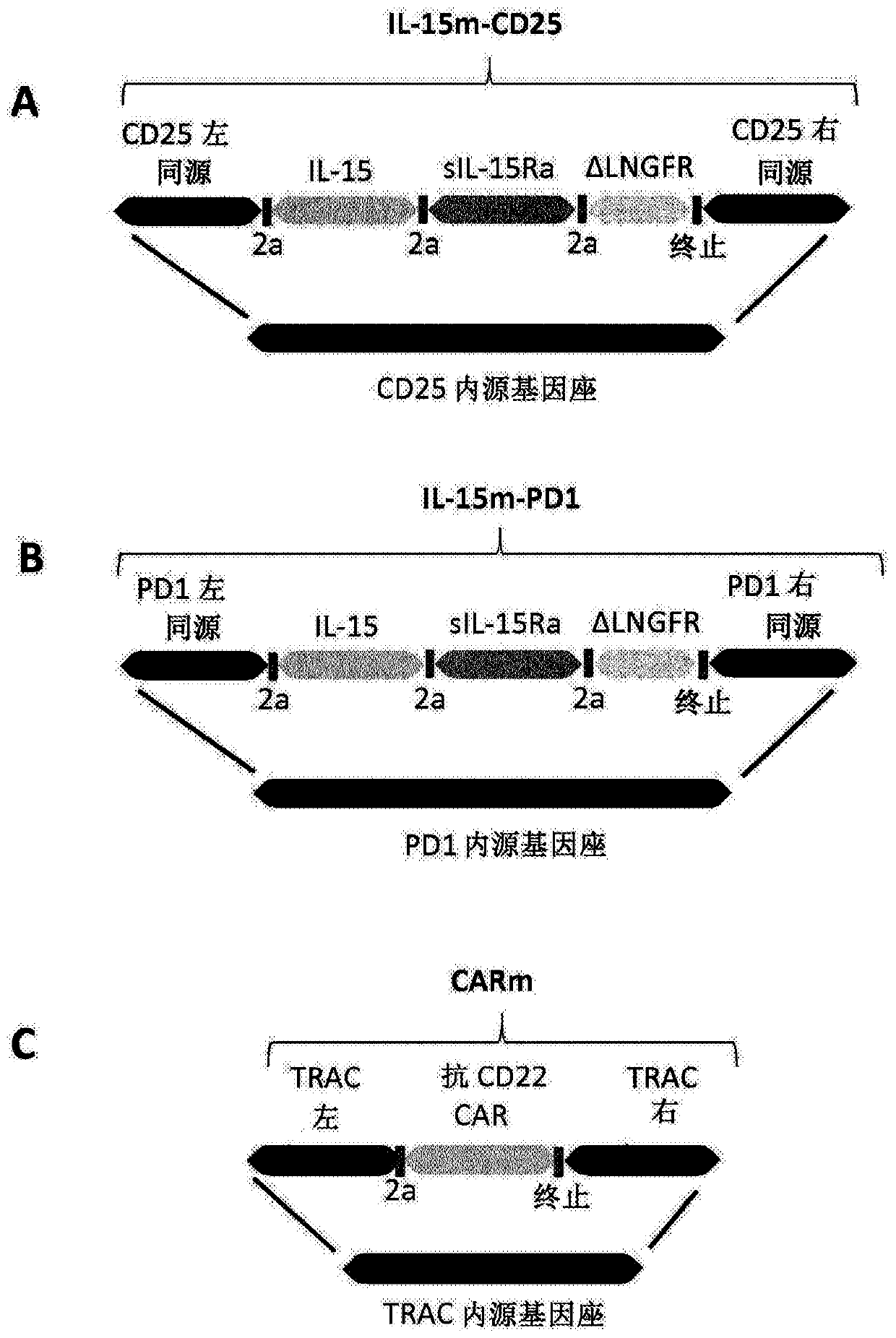
[0571] Example 3: Inhibition of NK inhibitors and CAR-encoding substrates at the B2M and TRAC loci in primary T cells -mediated dual targeting integration
[0572] This example describes methods to improve the therapeutic outcome of CAR T cell therapies by prolonging their in vivo persistence. The method consists in simultaneous B2M and TCR in the presence of an AAV6 repair vector delivering a CAR at the TRAC locus and an NK inhibitor at the B2M locus. mediated knockout. This approach prevents CAR T cells from attacking host tissues in a nonspecific and TCR-mediated manner (graft-versus-host attack) and diverts host T- and NK-cell-mediated depletion of CAR T cells.
[0573] The method developed to integrate NK inhibitors at the B2m locus lies in the presence of a DNA repair substrate vectorized by AAV6 using A double-strand break occurs in one of the first B2M exons. The matrix consists of two B2M homology arms embedded in the NK inhibitor coding sequence separated by 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com