CAR-T cell, preparation method thereof and medicine
A cell and dead cell technology, applied in the field of cell biology, can solve the problems of large side effects and weak tumor cell killing ability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0039] The present invention relates to a preparation method of CAR-T cells, comprising the following steps:
[0040] (1) Inoculate T cells in CAR-T medium and culture for 18-24 hours;
[0041] (2) introducing the vector carrying the chimeric antigen receptor sequence and the puromycin resistance gene into the cells obtained in step (1), and culturing for 18 to 24 hours;
[0042] (3) Collect the cells obtained in step (2) with 0.5~1×10 6 Cell density per ml was inoculated in CAR-T medium and cultured for 36-48 hours;
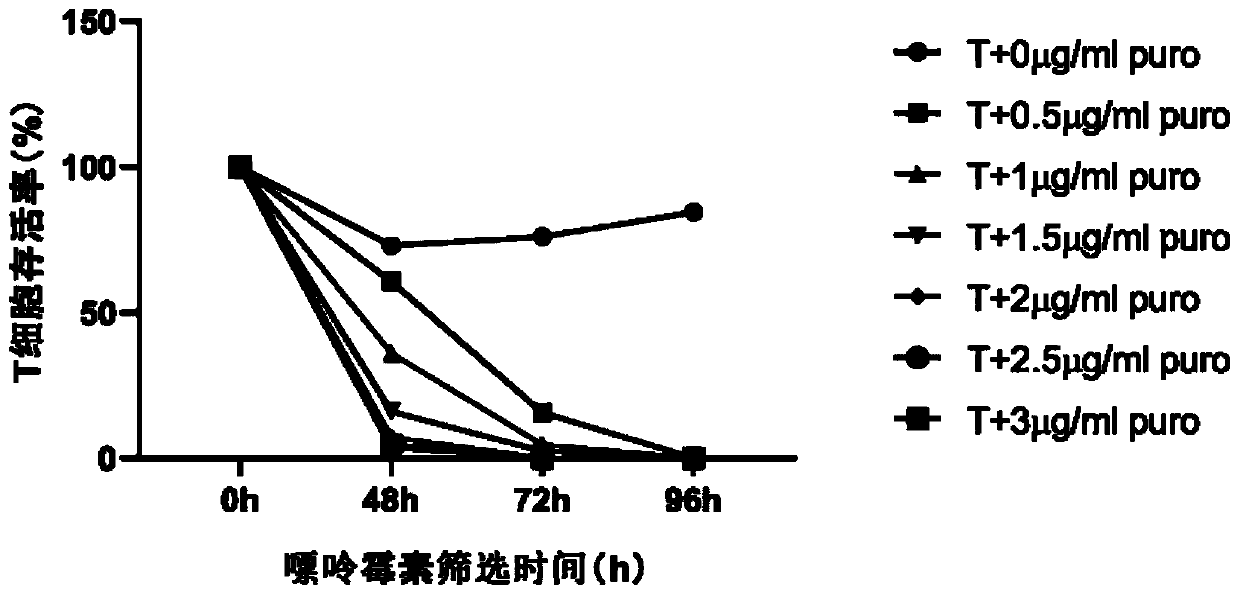
[0043] (4) Collect the cells obtained in step (3) with 0.5~1×10 6 Cell density per ml was inoculated in CAR-T medium, and puromycin was added, and cultured for 36-48 hours;
[0044] (5) Collect the cells obtained in step (4) and remove the dead cells therein, with 0.5~1×10 6 Cell density per ml was inoculated in CAR-T medium, and puromycin was added, and cultured for 36-48 hours to obtain CAR-T cells.
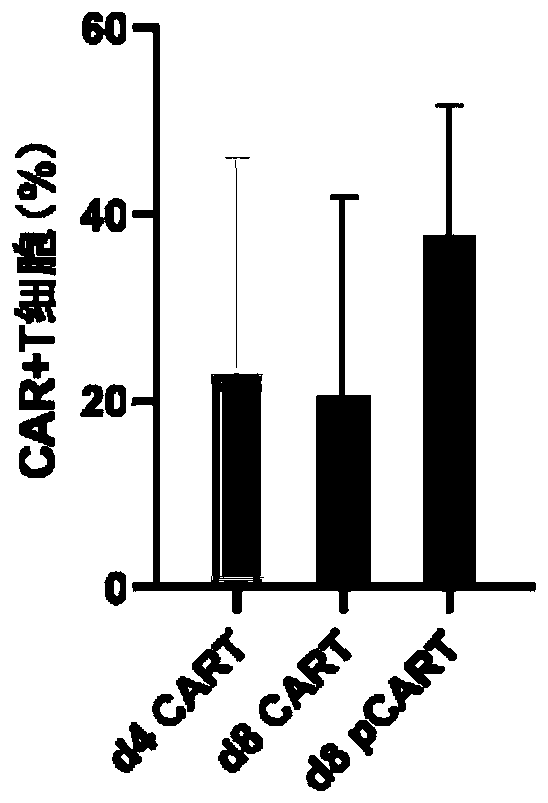
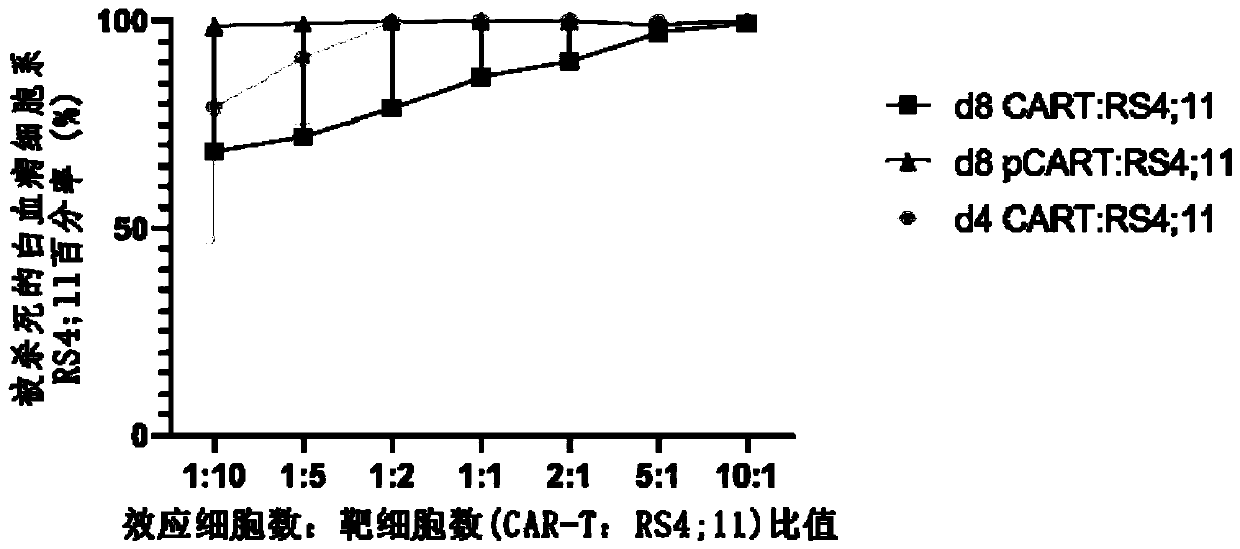
[0045] In the past studies, CAR-T cells cultured for 8...
Embodiment 1
[0056] Example 1 (d8pCAR-T)
[0057] Obtain 10mL peripheral whole blood from healthy donors or leukocyte apheresis to obtain leukocyte collection, use the kit RosetteSep Human T Cells enrichment cocktail from STEMCELL Technologies, operate according to the instructions, separate from peripheral whole blood or leukocyte collection with a purity of More than 99% of CD3+ T cells are frozen in liquid nitrogen or used directly.
[0058] D0 day: Take a certain amount of CD3+ T cells, use CAR-T medium (serum-free medium + 3% CTS TM ImmuneCell SR + 1% non-essential amino acid + 1% HEPES + 1% sodium pyruvate + 1% streptomycin-penicillin + 1% GlutaMAX + 100Unit / ml IL-2) for culture, and add Invitrogen's magnetic beads Dynabeads Human For T-Activator CD3 / CD28, the adding ratio is the number of cells: the number of magnetic beads = 2:1. at 37°C, 5% CO 2 Cultivate in an incubator for 18h to 24h.
[0059] Day D1: Use TAKARA's fibronectin solution to coat a six-well culture plate at room...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com