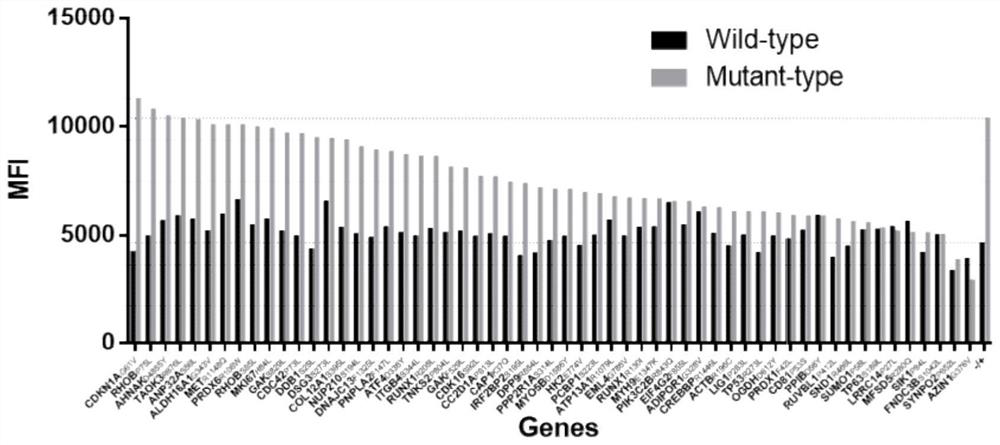
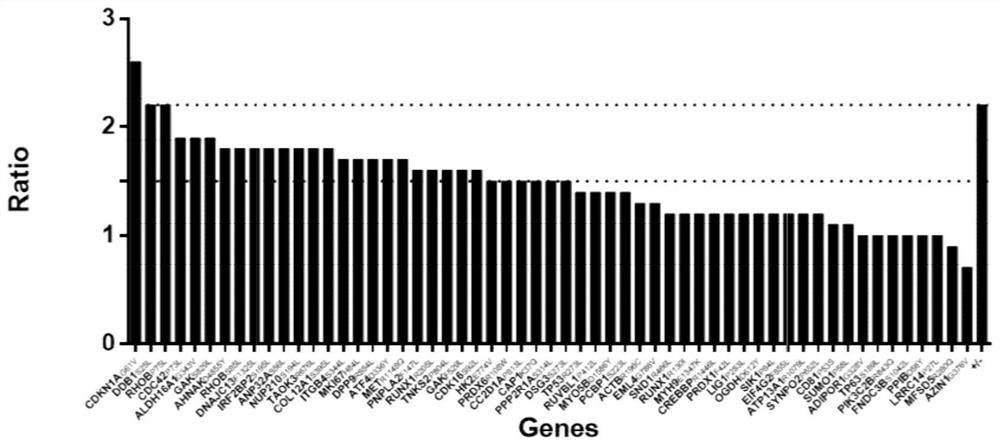
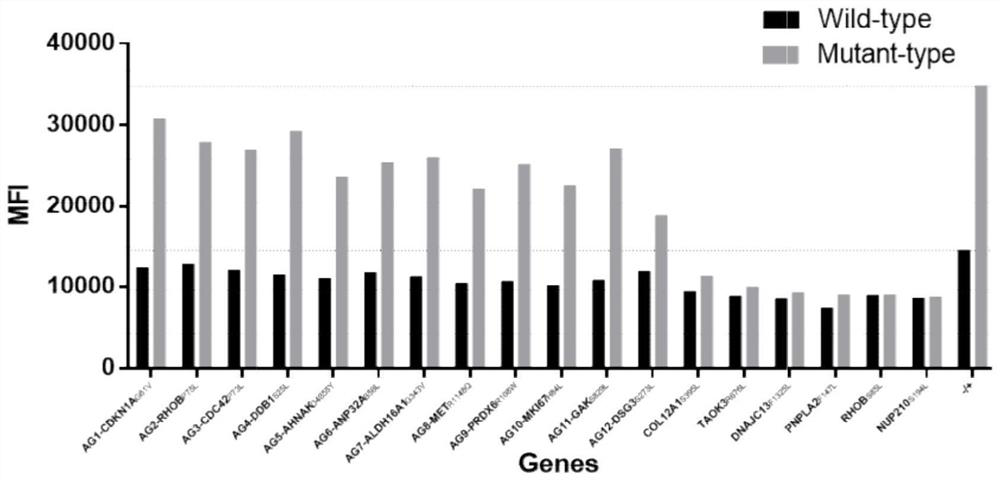
hla-a2-restricted bladder cancer neoantigen peptide sequence and its application
A HLA-A2, 1.HLA-A2 technology, applied in the HLA-A2-restricted bladder cancer tumor neoantigen peptide sequence, the application field of preparing bladder cancer diagnostic reagents and bladder cancer treatment drugs, can solve the problem of high degree of personalization , low mutation rate of tumor neoantigen peptides, high treatment costs and other issues, to achieve the effect of improving treatment effects, reducing treatment costs and increasing coverage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0050] The present invention will be described more specifically by way of examples below. It should be understood that the embodiments described here are used to explain the present invention, not to limit the present invention.
[0051] 1. Experimental materials:
[0052] 1. T2 cell line
[0053] T2 cells are B lymphoblastoid immortal cell lines transfected with human leukocyte antigen chimeric molecules. They cannot synthesize β2 microglobulin by themselves, and the expression of human leukocyte antigen on the cell surface is unstable. When exogenous β2 microglobulin is added Exogenous antigenic peptides can be presented to the cell surface to form a stable human leukocyte antigen-antigen peptide complex. Introduced by Stanford University in the United States.
[0054] 2. Reagents
[0055] Antigenic peptide: Shanghai Sangong
[0056] Ficoll separation liquid: Alere Technologies AS 1114547
[0057] 1640 Medium: Gibco
[0058] Fetal bovine serum: Gibco
[0059] β2 mic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com