A composition capable of sensitizing NK cells and its application
A technology of NK cells and drugs, which can be used in drug combinations, medical preparations with non-active ingredients, and medical preparations containing active ingredients, etc. It can solve the problems of cytotoxicity of immune cells, inhibit the potential of immunotherapy, etc., and achieve immunity. Inhibitory effect, promote immune killing effect, enhance expression effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Example 1 Preparation of nanoemulsion loaded with selenocystine and TGF-β inhibitor
[0056] 1. Preparation
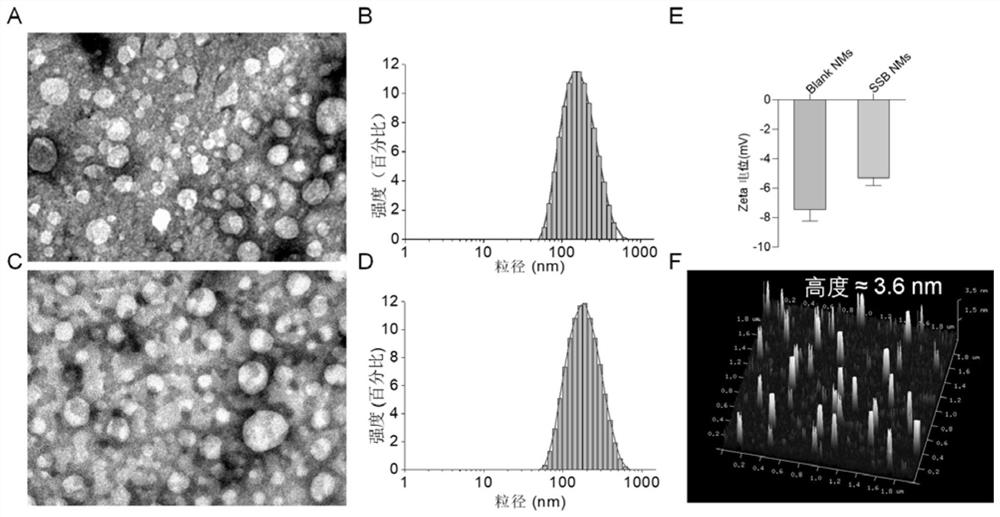
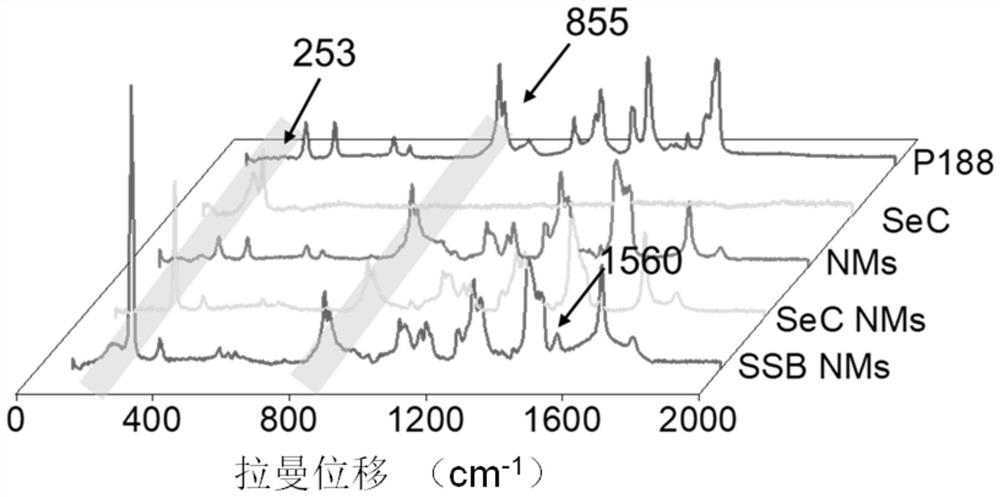
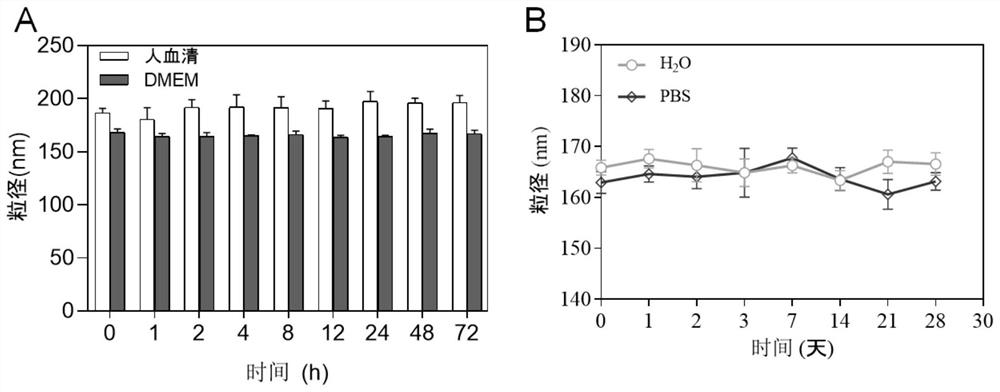
[0057] Weigh 2.0g of Poloxamer 188, 2.4g of olive oil, 2.4g of absolute ethanol, and 10mg of SeC into a beaker, then add 100μL of the prepared SB505124 stock solution (10mM), and stir at room temperature at 600rpm until the system becomes homogeneous phase, then drop by drop into 10mL of deionized water, and continue to stir for 30min to prepare colostrum. Subsequently, the prepared colostrum was transferred to a high-pressure homogenizer for homogenization step by step: first at a homogenization pressure of 250 Bar, homogenization for 3 minutes, and a sampling frequency of 40 Hz; then high-pressure homogenization, that is, the homogenization pressure was Homogenize for 20 minutes under the condition of 1200Bar, and the sampling frequency is 40Hz, and collect samples after homogenization. No SeC and SB505124 were added during the preparation process, and the o...
Embodiment 2
[0065] Embodiment 2 screening model drug test
[0066] NK cells and T cells extracted from tumor patients: 50 mL of peripheral blood was collected from the peripheral blood of tumor patient volunteers in a tertiary hospital in Guangdong, and peripheral blood mononuclear cells (PBMC) were extracted by Ficoll density gradient method; the cells were suspended in fresh ALYS505NK -In AC medium, adjust the density to 2×10 6 cells / mL, cultivated in a cell culture box for one day; then add 1000 U / mL recombinant human interleukin-2 (rhIL-2) and 10% autologous plasma to the cell culture medium, and continue to incubate for one day; the third day Add 30 mL of fresh ALYS505NK-EX medium, add 1000 U / mL rhIL-2 and rhIL-5, and incubate for two days; add 60 mL of fresh ALYS505NK-EX culture medium on the fifth day, add 1000 U / mL rhIL-2, and continue to incubate for 2 days; On the 7th to 14th day of culture, according to the color change of the culture medium, add double fresh serum-free medium...
Embodiment 3
[0076] Example 3 The Study of SSB NMs Stimulating NK92
[0077] (1) The combined effect of SSB NMs and NK92 and the ratio of NK92 cells added are set as follows:
[0078] Group 1: After pre-incubating different proportions of NK92 cells with different concentrations of SSB NMs for 12 hours, the two were added to MDA-MB-231 cells at the same time; the drug concentrations were 1 μM, 4 μM, 8 μM, 16 μM and 32 μM, and The addition amount was 100 μl / well. Tumor cells were counted according to the number when they were plated, and the amount when plated was 4×10 3 pcs / well (96-well plate). Effector cell:tumor cell (E:T) ratios were 2.5:1, 5:1, 10:1 and 20:1, respectively.
[0079] Group 2: MDA-MB-231 cells were pretreated with different concentrations of SSB NMs for 12 hours, and then NK92 cells were added in different proportions; the concentration of drugs and the ratio of effector cells: tumor cells were set the same as in Group 1.
[0080] Group 3: Different concentrations of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| height | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com