A novel coronavirus S protein single-region subunit nanovaccine based on Helicobacter pylori ferritin
A technology of Helicobacter pylori and coronavirus, applied in the directions of viruses, viral peptides, antiviral agents, etc., to achieve the effects of easy purification, simple preparation method, and overcoming insufficient immunogenicity of monomers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] Embodiment 1 constructs novel coronavirus SARS-CoV-2 antigen (fusion protein RBD-HP_Ferritin)
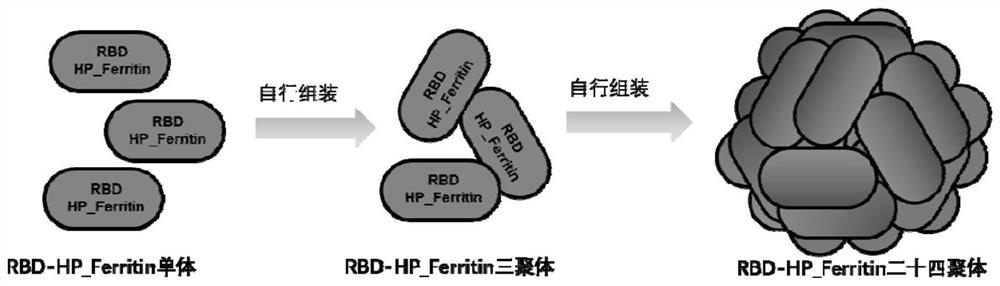
[0075] The schematic diagram and structural diagram of fusion protein RBD-HP_Ferritin self-assembled nanoparticles are as follows figure 1 and figure 2 shown.
[0076] Specifically, the construction and preparation method of the fusion protein RBD-HP_Ferritin is as follows:
[0077] 1. Preparation of vector expressing RBD-HP_Ferritin antigen
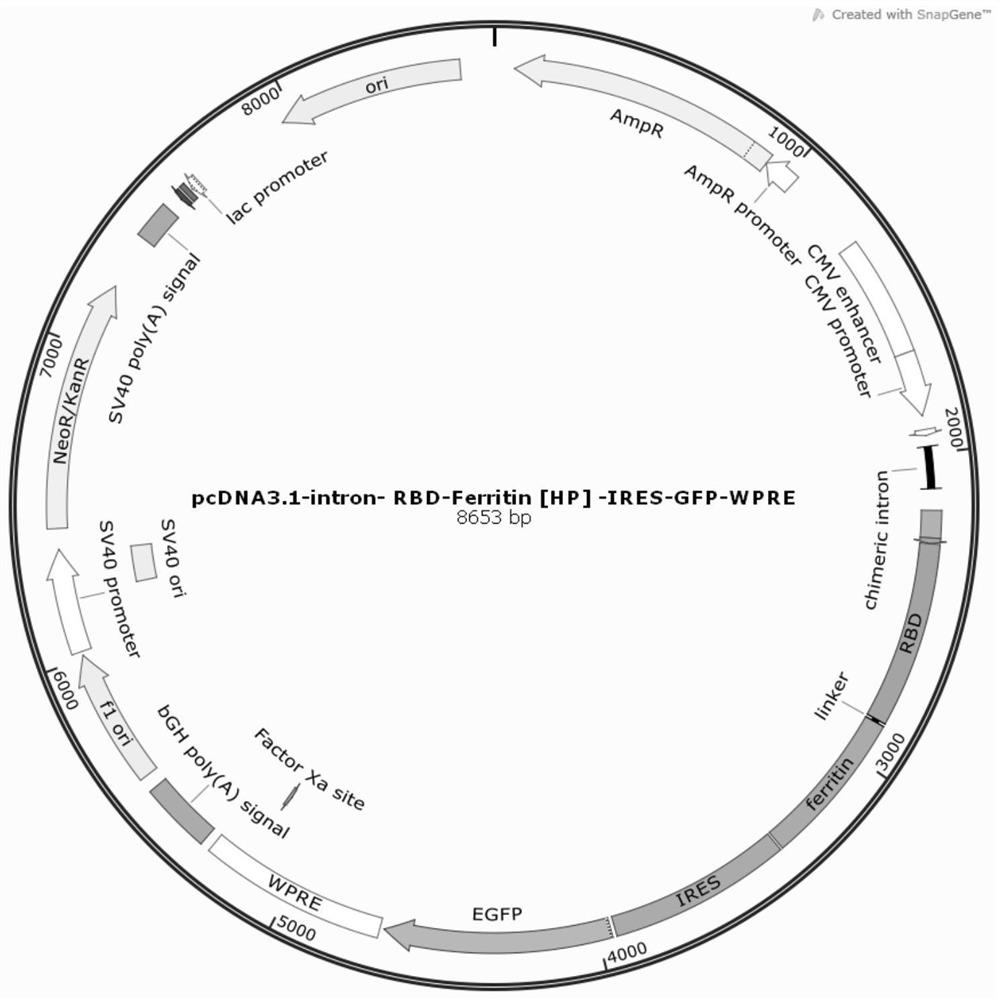
[0078] After the 3' end of the RBD-HP_Ferritin nucleotide sequence is added with a translation stop codon, it is cloned between the Xho I and Xba I restriction sites of the expression vector (pcDNA3.1-Intron-WPRE) that adds Intron and WPRE to enhance expression , construct the expression vector pcDNA3.1-Intron-WPRE-RBD-Ferritin (HP)-IRES-GFP (such as image 3 shown).
[0079] DH5αcompetent cells were transformed with the recombinant plasmid, cultured overnight at 37°C, and positive clones were identified by screening and PCR. Ext...
Embodiment 2
[0084] Embodiment 2 mouse immunization experiment
[0085] The RBD-HP_Ferritin antigen obtained in Example 1 was diluted to 100 μg / ml with physiological saline according to Table 1, and emulsified in groups with an equal volume of adjuvant SAS. Then 6-8 weeks old Balb / C mice were immunized in groups. immunization strategies such as Figure 8 As shown, that is, by intraperitoneal injection, each mouse received 3 vaccine immunizations on the 0th day, the 3rd week (21 days), and the 14th week (108 days), each inoculation volume of 200 μl (10 μg) . On the 10th, 31st, and 108th days, blood was collected from the mice's orbits. The mouse serum was obtained by centrifuging at 2800rpm for 15 minutes at 4°C after standing for a period of time to allow the serum to separate out, and was immediately used in the SARS-CoV-2 pseudovirus neutralization detection experiment.
[0086] Table 1
[0087] Antigen / Control Antigen content Adjuvant Number of animals (only) R...
Embodiment 3
[0088] Embodiment 3 Pseudovirus neutralization test
[0089] 1. Preparation of pseudovirus:
[0090] According to the sequence published by NCBI, the Spike protein of SARS-CoV-2 was synthesized and inserted into the pcDNA3.1 expression vector. The expression vector of SARS-CoV-2 Spike protein was co-transfected into 293T cells with pHIV-luciferase and psPAX2 plasmids. After 5 hours of transfection, the cells were washed twice with PBS and replaced with serum-free DMEM medium to continue culturing. After 48 hours the supernatant was harvested and centrifuged to remove cell debris. Afterwards, the HIV-luc / SARS-CoV-2-S pseudovirus was obtained by dissolving with a small volume of serum-free DMEM.
[0091] The pseudovirus can effectively simulate the process of wild-type SARS-CoV-2 invading cells. When it infects production cells or target cells, the expression of the luciferase reporter gene carried by the SARS-CoV-2 pseudovirus can accurately reflect the results of virus infe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com