Materials and methods including for sex selection
A male, optional technology, applied in the direction of chemical apparatus and methods, botany apparatus and methods, biochemical apparatus and methods, etc., which can solve the problem of suboptimal success rate, lack of reproducibility, lack of consistency of IVP protocol, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] Example 1 - Simplified IVP method that essentially uses a single medium to increase yield of porcine embryos
[0081] This example describes a simplified method for pre-implantation in vitro porcine embryo development suitable for high-throughput IVP systems.
[0082] Materials and methods
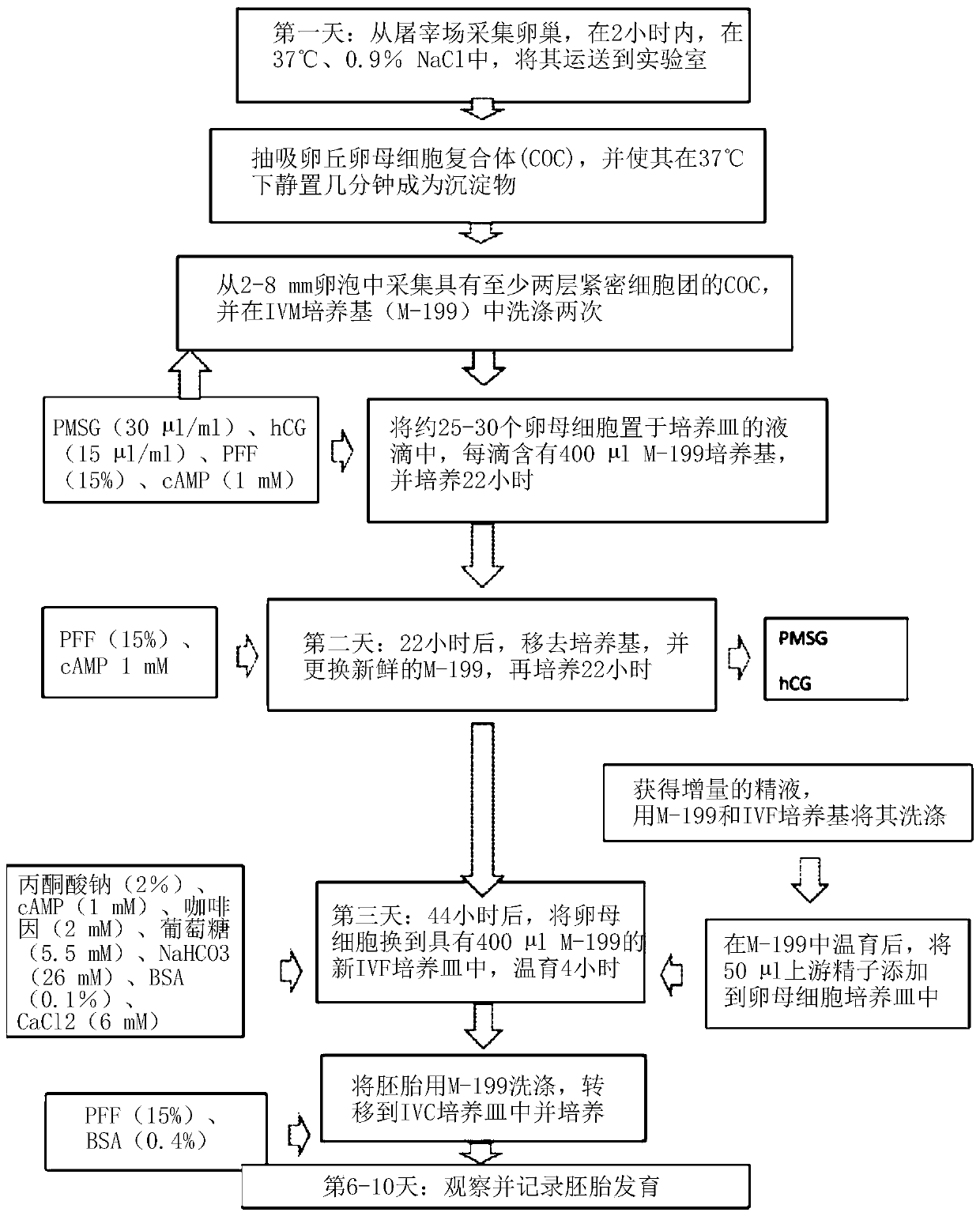
[0083] All chemicals were purchased from Thermo Fisher Scientific unless otherwise stated. All in vitro cultures were prepared by overlaying 400 μl of mineral oil in each culture well. The basal medium used in this example was M-199. In 1950, Morgan first proposed a chemically defined source of nutrients for cell culture without the use of animal products and / or tissue extracts. The advantage of using this medium is that it has a wide variety of components to support the development of oocytes and embryos at different developmental stages.
[0084] Oocyte recovery and in vitro maturation
[0085]Ovaries were collected from slaughtered prepubertal gilts and sows from a nearby sla...
specific Embodiment approach
[0209] Preferred features, embodiments and modifications of the invention can be seen from the following detailed description, which provides those skilled in the art with sufficient information to practice the invention. The detailed description should not be considered as limiting the scope of the foregoing summary in any way. The detailed description will refer to the following several drawings:
[0210] Figure 1B : Amplification profile of the Y chromosome, indicating thresholds and wells with Y chromosome amplicons.
[0211] Figure 2B : Amplification profile of the 12S chromosome, indicating thresholds and wells with 12S chromosome amplicons.
[0212] Figure 3B : Amplification profile of 12S chromosome and Y chromosome (Chr Y) combination, indicating threshold and wells with 12S chromosome and Y chromosome amplicons.
specific Embodiment approach 2
[0214] Example 1 - Differential lysis of embryos or eggs in the presence of sperm or semen for polymerase amplification.
[0215] This example describes the use of a novel lysis buffer / solution for differentially lysing embryos or eggs in the presence of sperm or semen for PCR amplification. DNA from individually lysed embryos or eggs can then be pre-amplified by PCR to generate sufficient whole-genome DNA for a variety of downstream applications.
[0216] The high-throughput method described here is a rapid, 25-minute, single-tube process that is delicate enough to selectively lyse oocytes in the presence of spermatozoa, yet sensitive enough to detect ovum from a single unfertilized egg and those that have developed. Embryos of 2, 4, 8, 16 or more cells are lysed and DNA preamplified. Furthermore, no substances were introduced that might inhibit preamplification or downstream applications. The method is also compatible with the Single Cell REPLIg Kit as well as other qPCR a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com