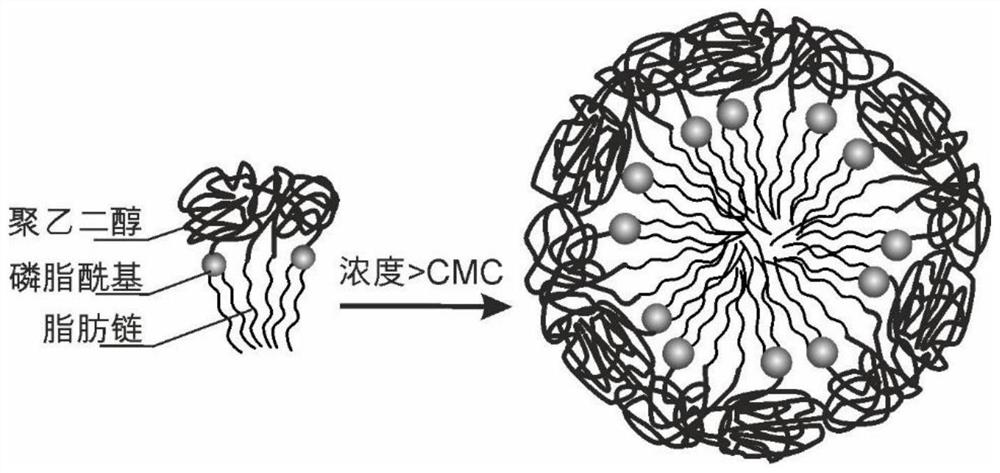
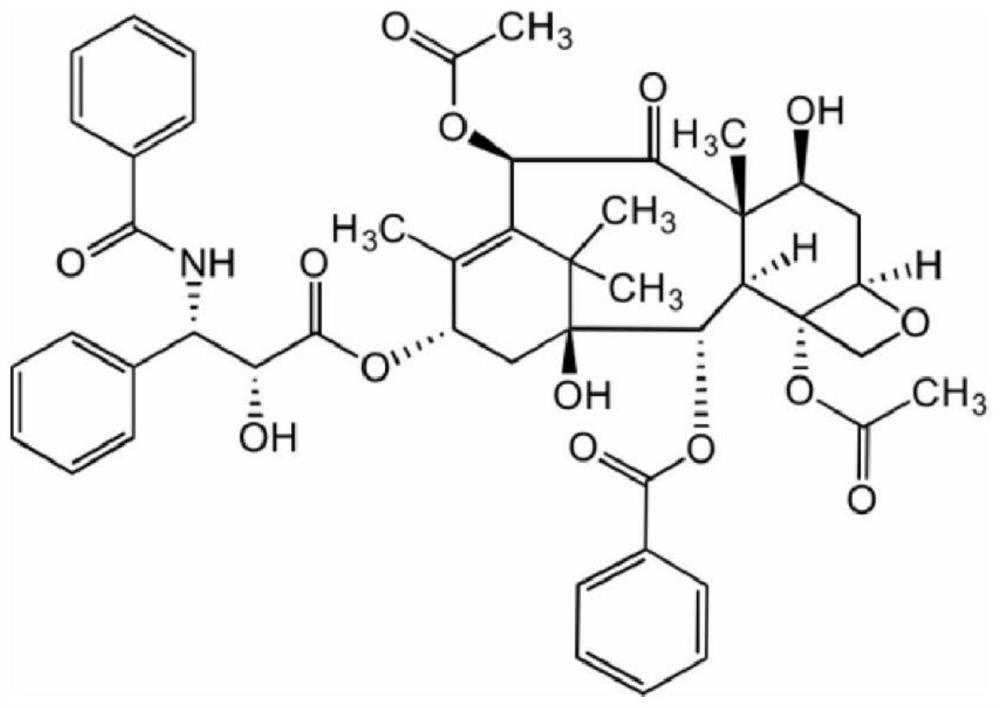
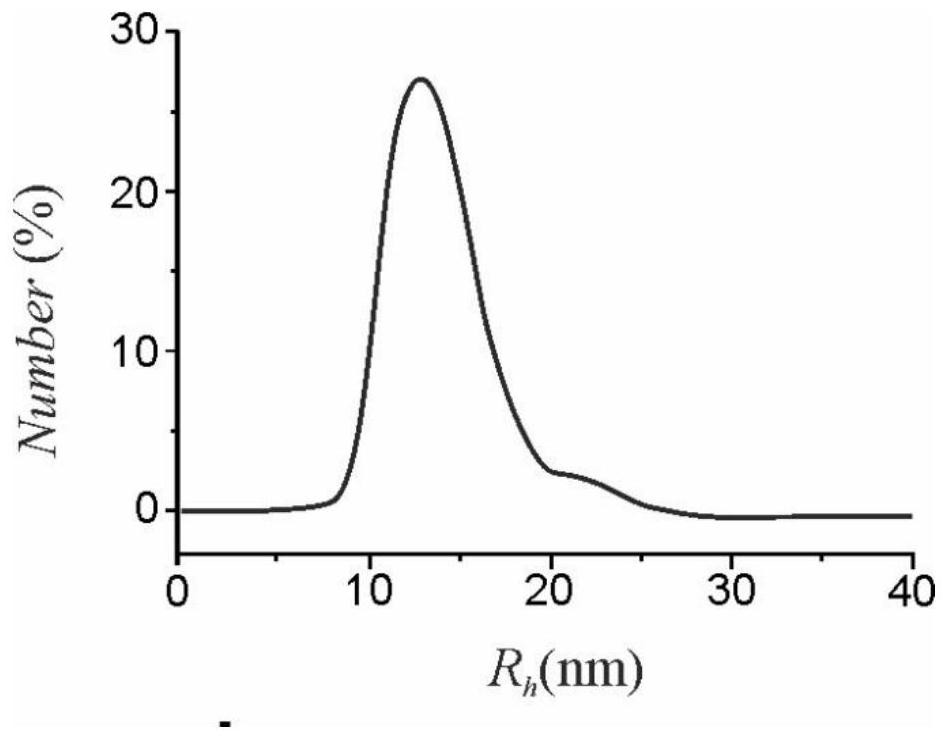
Application of micelles formed by polyethylene glycol derivatives in paclitaxel or derivatives thereof
A polyethylene glycol, polyethylene glycol group technology, applied in non-active ingredients medical preparations, medical preparations containing active ingredients, drug combinations, etc. The distribution and number of hydrophobic chains are difficult to precisely control, reducing tissue penetration and other problems, achieving long-term stability and controllability, overcoming low organic solvent tolerance, and highlighting the effects of biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0099] The preparation method of above-mentioned polyethylene glycol derivative, described method comprises the steps:
[0100] (a) phospholipid compound, (b) polyethylene glycol PEG-(Y) with Y terminal group n , and optionally (c) fatty chain-containing compound R 4 -X 1 Mix and react to obtain the polyethylene glycol derivative.
[0101] In one embodiment of the present invention, the above raw materials are mixed in a solvent, for example, the solvent is selected from N,N-dimethylformamide.
[0102] In one embodiment of the present invention, described method comprises the steps:
[0103] (1) (a) phospholipid compound and optional (c) fatty chain-containing compound R 4 -X 1 Dissolved in a solvent to obtain a mixed solution A;
[0104] (2) the polyethylene glycol PEG-(Y) whose end group is Y in (b) n Dissolved in a solvent to obtain a mixed solution B;
[0105] (3) Mix the mixed solution A and the mixed solution B, and react at room temperature to prepare the polyet...
preparation example 1 3
[0120] Preparation Example 1 The synthesis of three aliphatic chain-polyethylene glycol derivatives (1DSPE, 1 n-octadecyl mercaptan)
[0121] Weigh 50 mg of DSPE in a 10 mL round-bottom glass flask, dissolve it in 100 μL of N,N-dimethylformamide, and add 12 μL of diisopropylethylamine to obtain a solution (1).
[0122] Take by weighing 334mg end group substituted polyethylene glycol OPSS-PEG5000-SCM (average molecular weight 5000Da, end group is the polyethylene glycol of dithiopyridyl and succinimide carboxymethyl ester respectively with 2 milliliters weighing bottle, (purchased from Beijing Jiankai Technology Co., Ltd., product number A5109), and dissolved in 400 μL DMF to obtain solution (2).
[0123] The solution (2) was added into the round-bottomed flask with the solution (1) for mixing, stirred by magnetic force, and reacted at room temperature for 4 hours under the protection of nitrogen. Thereafter, nitrogen protection was removed, and 100 μL of 0.5 M hydrochloric ac...
preparation example 2 3
[0125] Preparation Example 2 The synthesis of three aliphatic chain-polyethylene glycol derivatives (1DSPE, 1 n-octyl mercaptan)
[0126] Weigh 50 mg of DSPE in a 10 mL round bottom glass flask, dissolve it in 100 μL of DMF, add 12 μL of diisopropylethylamine to obtain solution (1).
[0127] Take by weighing 334mg end group substituted polyethylene glycol MAL-PEG5000-SCM (average molecular weight 5000Da, end group is respectively the polyethylene glycol of maleimide and succinimide carboxymethyl ester) with 2 milliliter weighing bottle , purchased from Beijing Jiankai Technology Co., Ltd., product number A5003), which was dissolved in 400 μL DMF to obtain solution (2).
[0128] Add 15 μL of 1-octanethiol (molecular weight 146, purchased from Beijing Bailingwei Technology) to solution (2), and react for 1 hour under nitrogen protection. The obtained reaction liquid was added into the round bottom flask with solution (1) for mixing, stirred by magnetic force, and reacted under ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| number average molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com