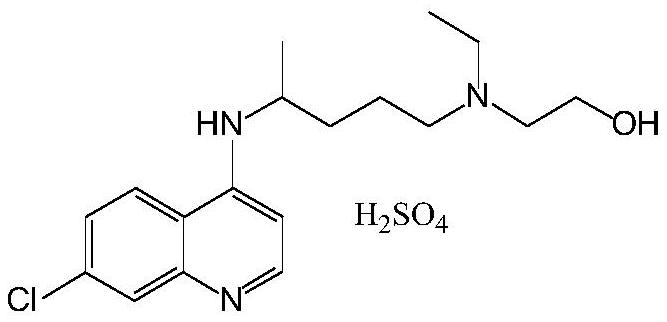
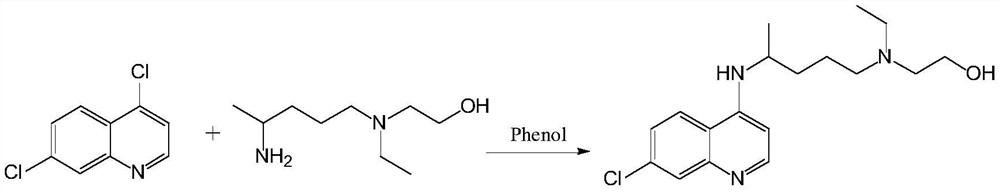
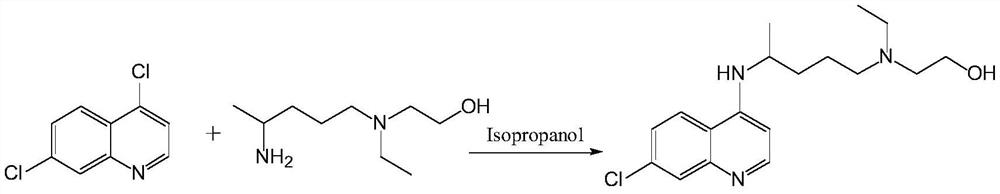
A kind of preparation method of hydroxychloroquine
A technology of hydroxychloroquine and hydroxyquinoline, which is applied in the field of preparation of hydroxychloroquine, can solve the problems affecting the final quality of the product, unfavorable industrial application, high cost, etc., and is beneficial to large-scale production, prevents the generation of isomers, and is not easy to produce. The effect of side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] The HPLC purity is 99.73%, and the largest single impurity is 0.08%.
Embodiment 2
The ethyl acetate solution was removed, washed with water, and concentrated to obtain the crude product. Isopropanol was recrystallized to obtain off-white crystals (30g) with a yield of
91%. Melting point: 91‑92℃.
The content of liquid chromatographic analysis hydroxychloroquine is 99.62%.
The preparation of hydroxychloroquine sulfate
[0077] According to the preparation process described in Example 1, hydroxychloroquinoline sulfate was obtained, and the melting point was 239 °C to 241 °C.
[0078] HPLC purity 99.79%, the largest single impurity is 0.06%.
Embodiment 3
The ethyl acetate solution was removed, washed with water, and concentrated to obtain the crude product. Isopropanol was recrystallized to obtain off-white crystals (32.3 g) in a yield of
96%. Melting point: 91-92℃.
The content of liquid chromatographic analysis hydroxychloroquine is 99.69%.
The preparation of hydroxychloroquine sulfate
[0084] According to the preparation process described in Example 1, hydroxychloroquinoline sulfate was obtained, and the melting point was 239 °C to 241 °C.
[0085] The HPLC purity was 99.87%, and the largest single impurity was 0.04%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com