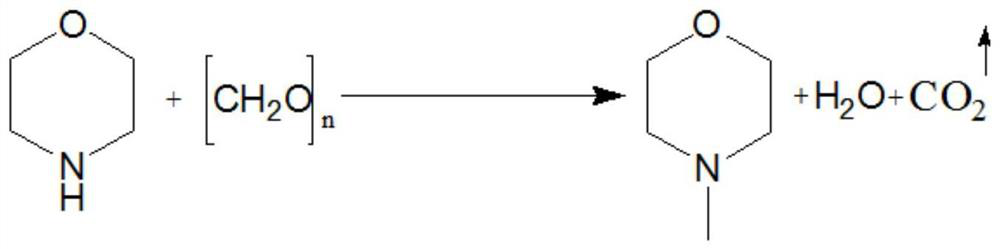
Synthesis process of N-methylmorpholine
A synthesis process and a technology for methyl morpholine, which are applied in the field of synthesis technology of N-methyl morpholine, can solve the problems that the catalyst preparation process is cumbersome, unsatisfactory, and the reaction temperature is high, so as to improve the industrial application prospect and simplify the Equipment and process requirements, the effect of simple reaction process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] as attached figure 1 The reaction formula is shown as:
[0048] 50g morpholine, 25g water are joined in the reactor;
[0049] The temperature of the reaction kettle was raised to 85°C, 55g of paraformaldehyde was added, stirred, and kept warm for 5 hours until no tail gas flowed out, and the reaction was completed.
[0050] Cool down to 30°C, add 10g of sodium chloride to the reaction kettle, the system is layered, the upper organic phase is rectified and purified, and the lower aqueous phase is used in the next batch to obtain N-methylmorpholine with a yield of 98.7% per pass, with a purity of 99.3%, as light yellow liquid.
[0051] After detection, its GC-MS analysis molecular weight is 101.15, through 1 H-NMR detection confirmed that it was N-methylmorpholine.
Embodiment 2
[0053] 50g morpholine, 28g water are joined in the reactor;
[0054] The temperature of the reaction kettle was raised to 85° C., 58 g of paraformaldehyde was added, and stirred and kept for 6 hours until no tail gas flowed out, and the reaction was completed.
[0055] Cool down to 30°C, add 10g of sodium chloride to the reaction kettle, the system is layered, the upper organic phase is rectified and purified, and the lower aqueous phase is used in the next batch to obtain N-methylmorpholine with a yield of 99.2% per pass, with a purity of 99.5% %, it is light yellow liquid.
[0056] After detection, its GC-MS analysis molecular weight is 101.15, through 1 H-NMR detection confirmed that it was N-methylmorpholine.
Embodiment 3
[0058] Concrete operation is identical with embodiment 2, and difference is that morpholine, water, paraformaldehyde join in the reactor simultaneously.
[0059] Yield per pass is 87.3%, purity 98.3%, it is light yellow liquid. At the same time, 4.3% N-formylmorpholine was obtained by separation.
[0060] After detection, its GC-MS analysis molecular weight is 101.15, through 1 H-NMR detection confirmed that it was N-methylmorpholine.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com