Application of miR-486-3p to preparation of product for treating neuroinflammation caused by SAH
A technology of neuroinflammation and products, applied in the application field of neuroinflammation products, can solve problems such as difficult to achieve clinical effects and unable to cross the BBB
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1, qRT-PCR verifies NGS data
[0035] 1. Extract the total RNA of exosomes by QIAzol-spin column method, measure the purity of RNA, and quantify the RNA. Using the corresponding solvent as the control (Blank), take 2 μL of the RNA solution and detect it in Merinton SMA4000, and observe A 260 / A 280 、A 260 / A 230 Ratio and continuous wavelength absorption peak, and calculate the concentration of RNA solution to judge the quality of RNA extraction: A 260 / A 280 >2.0 and <2.3, it can meet the requirements of subsequent RT-qPCR. Total RNA was extracted from exosomes or brain tissue using QIAzol Lysis reagent (Qiagen, Germany).
[0036] Table 1
[0037]
[0038]
[0039] 2. Quantitative PCR of miRNA: To analyze miRNA levels, total RNA was reverse-transcribed into cDNA using miRcute miRNA FirstStrand cDNA Synthesis Kit (Tiangen Biotechnology Company, China). Reverse transcription system preparation: total RNA 2μg, 2×miRNA RT Reaction Buffer 10μl, miRNA...
Embodiment 2
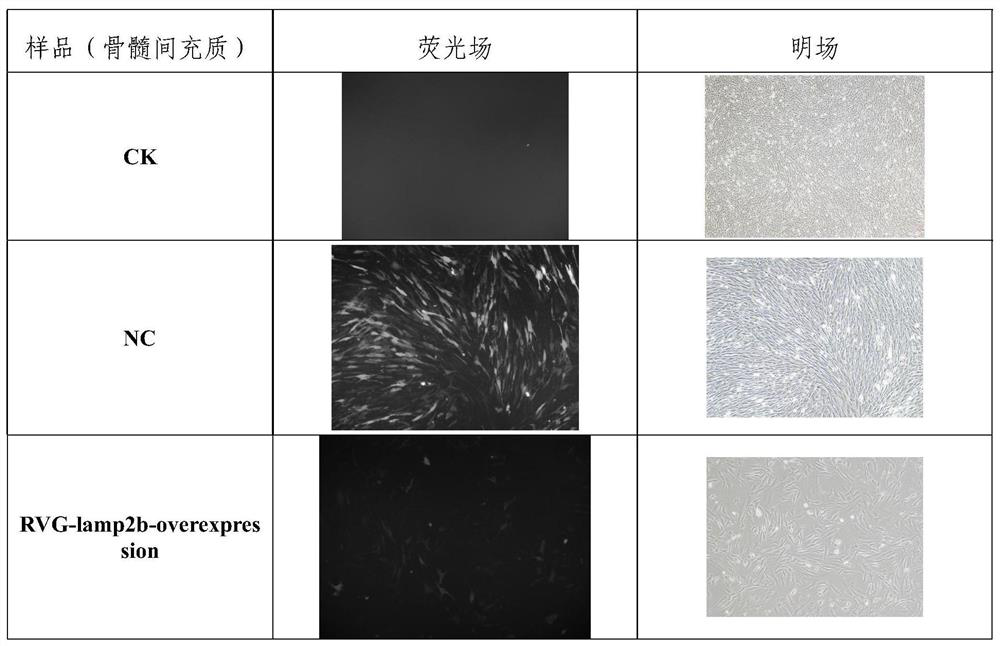
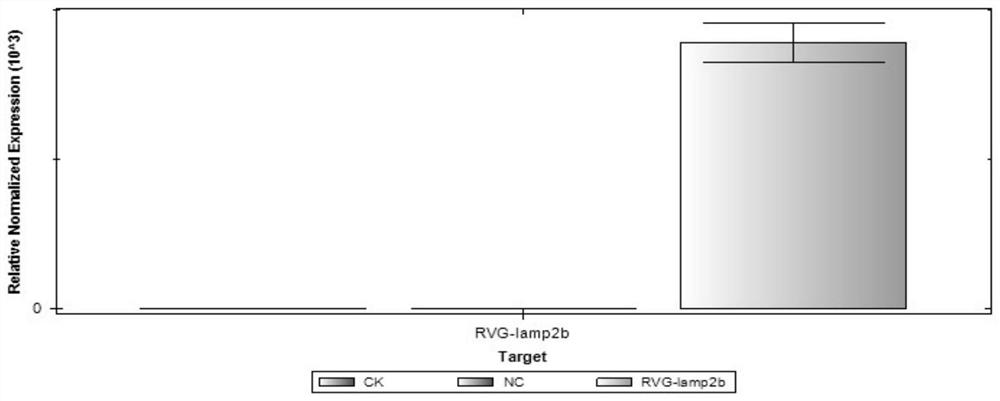
[0041] Example 2, RVG-LAMP2B-overexpression lentiviral vector construction
[0042] 1. Experimental method:
[0043] 1.1 Obtain the RVG-LAMP2B-mus sequence fragment by PCR method
[0044] 1.1.1 The whole gene synthesis of the vector was synthesized by Jierui Bioengineering Co., Ltd.
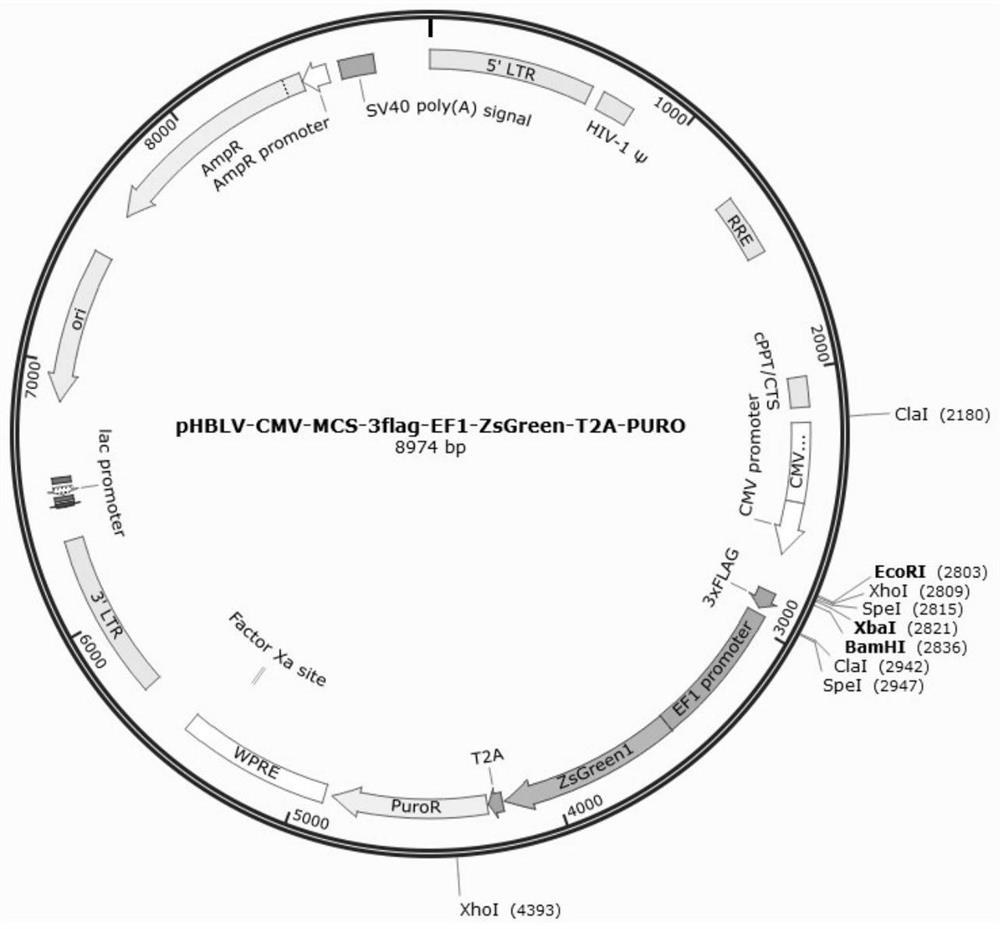
[0045] 1.1.2 Primers: Gene name: RVG-LAMP2B-mus; Cloning vector: pHBLV-CMV-MCS-3FLAG-EF1-ZsGreen-T2A-PURO, cloning vector such as figure 1 shown. Cloning strategy: BamHI+EcoⅠ. The upstream and downstream primers of the target gene were respectively added with homologous sequences on both sides of EcoI and BamHI on the PHBLV-CMV-MCS-3FLAG-EF1-ZSGREEN-T2A-PURO vector for subcloning of the vector. The primer sequences are as follows:
[0046] Primers were synthesized by Shanghai Hanbio Co., Ltd.
[0047] m-RVG-lamp2b-F: (SEQ ID NO.22)
[0048] agaggatcta tttccggtga attcgccacc atgtgcctct ctccggtta
[0049] m-RVG-lamp2b-R: (SEQ ID NO.23)
[0050] cacttaagct tggtaccgag gatcccagag tctgatatcc agc...
Embodiment 3
[0062] Example 3, RVG-LAMP2B-overexpression lentiviral packaging
[0063] 2. Experimental steps:
[0064] 2.1 Culture and transfection of 293T cells
[0065]When the 293T cells were cultured in a 10cm dish until 80-90% confluent, they were inoculated into a 15cm dish. Pour off the culture medium and wash the cells twice with 1mL D-Hank’s solution. Add 1mL Trypsin-EDTA solution, mix well, and place at 37°C for 2-3min.
[0066] Carefully suck off the trypsin solution, add 2 mL of DMEM culture solution containing 10% FBS, and pipette the cells to form a single-cell suspension. Inoculate the cell suspension into a 15cm culture dish, add 18mL DMEM culture solution containing 10% FBS, and mix well at 37°C in 5% CO 2 Incubate overnight.
[0067] Add 1.5mL serum-free DMEM to a sterile 5mL centrifuge tube, add shuttle plasmid V3120 and packaging plasmids (pGag / Pol, pRev, pVSV-G) in proportion, mix well, take another sterile 5mL centrifuge tube tube, add 1.5mL serum-free DMEM, the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com