Document management method and system for clinical trials
A clinical trial and document management technology, applied in the field of clinical trial data management, can solve the problems of low management efficiency, inability to classify and store documents, document editing information records, etc., to improve management efficiency, ensure non-repudiation, and achieve a high degree of automation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0081]The present invention will be described in detail below with reference to the specific embodiments shown in the drawings. However, these embodiments are not limited to the present invention, and those skilled in the art are included in the scope of the invention in accordance with the structures, methods, or functional transformations made in the present invention.
[0082]The specific embodiments are described as an example in which the clinical trial document management method and system provided by the present invention are not limited to pharmaceutical clinical trials, and the general clinical trial, such as medical instrument clinical trials. Clinical trial of in vitro diagnostic reagent.
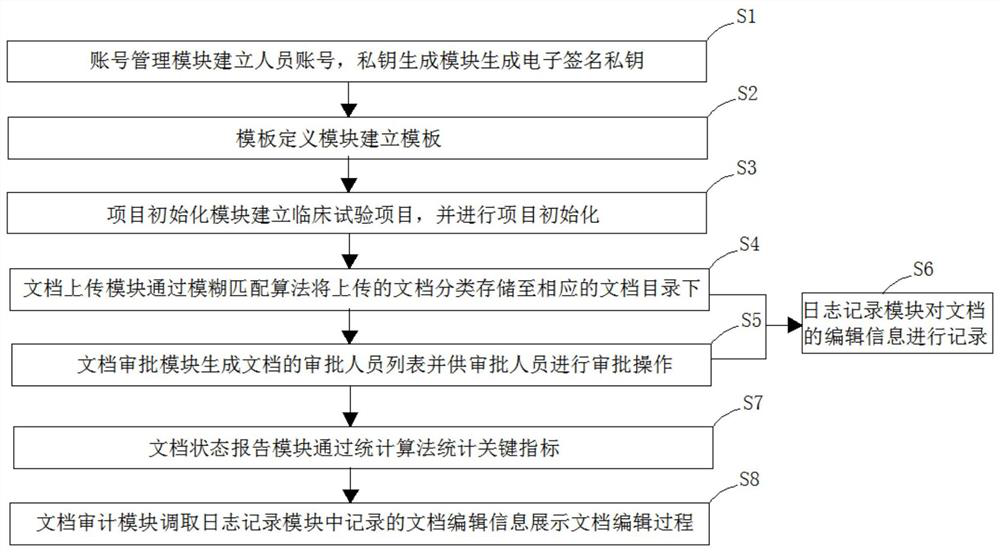
[0083]Seefigure 1 , A document management method for clinical trials, including the following steps:
[0084]S1, account management module establishes a person account, generates user account data, and uses account information to generate one or one corresponding electronic signature private ke...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com